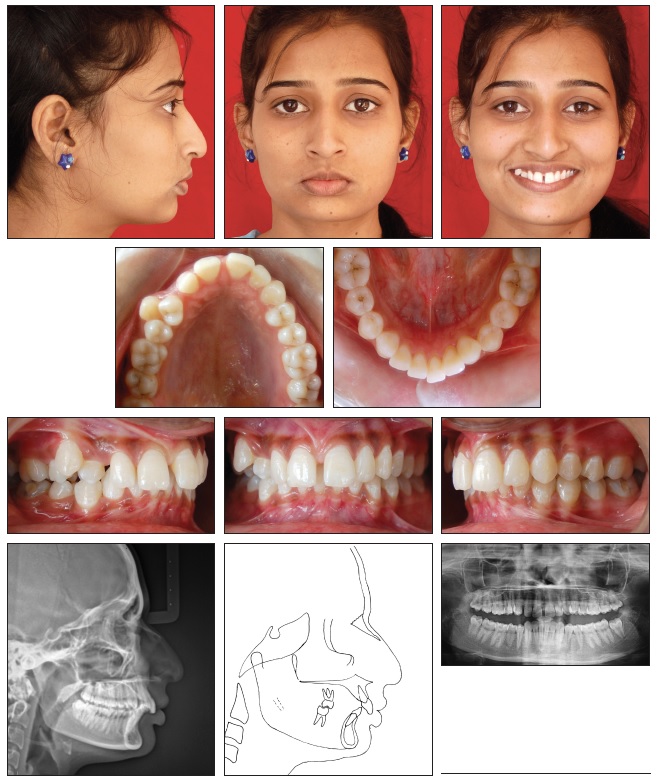
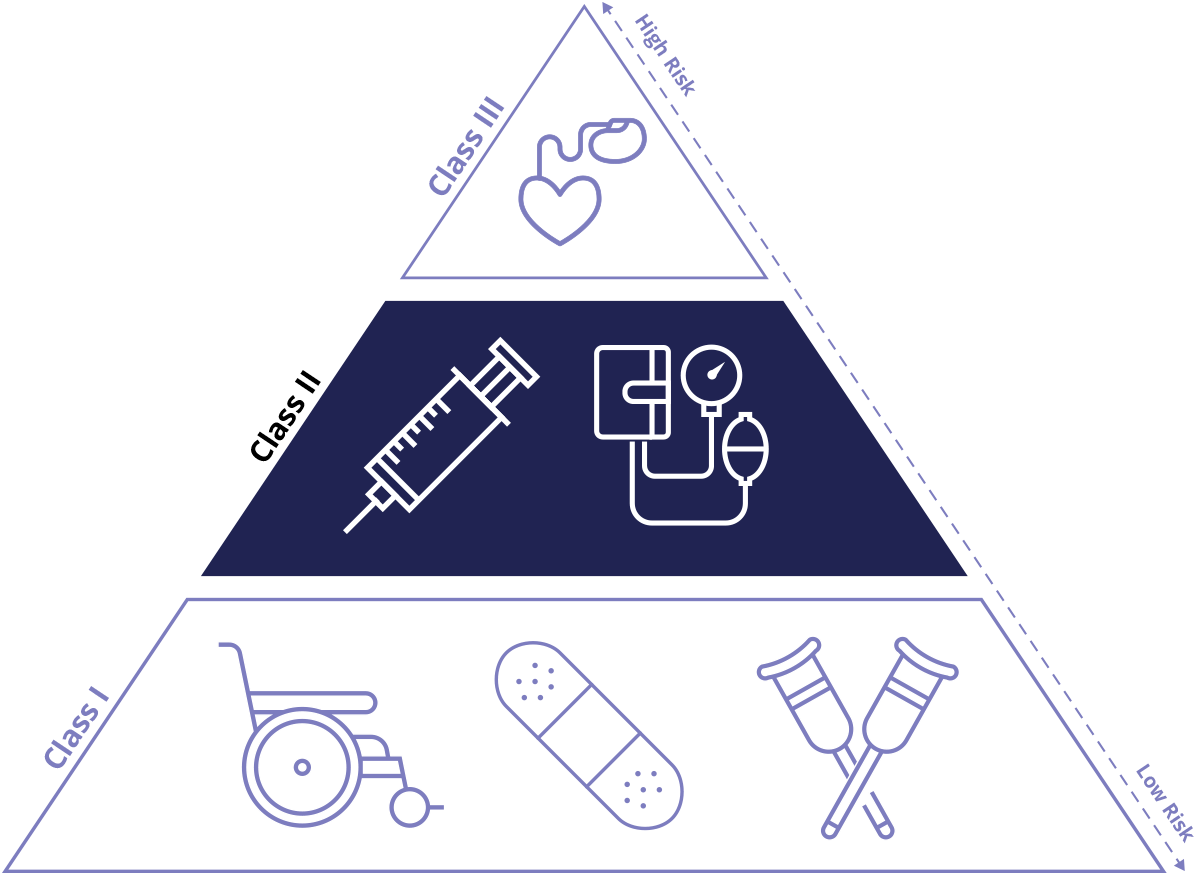
Class II medical devices have moderate to higher risks to patients or users. Over 40% of medical devices fall into this device category. The majority of medical devices are considered to be Class II devices. Some examples of Class II devices include catheters, syringes, contact lens, and pregnancy test kits.
A guide to FDA regulations for medical devices - Spyrosoft
The 3 FDA medical device classes: differences and examples explained

New world order 2013

Entering the US Market: Medical Devices - ppt video online download

Medical Devices; US and Chinese legislation - Kvalito
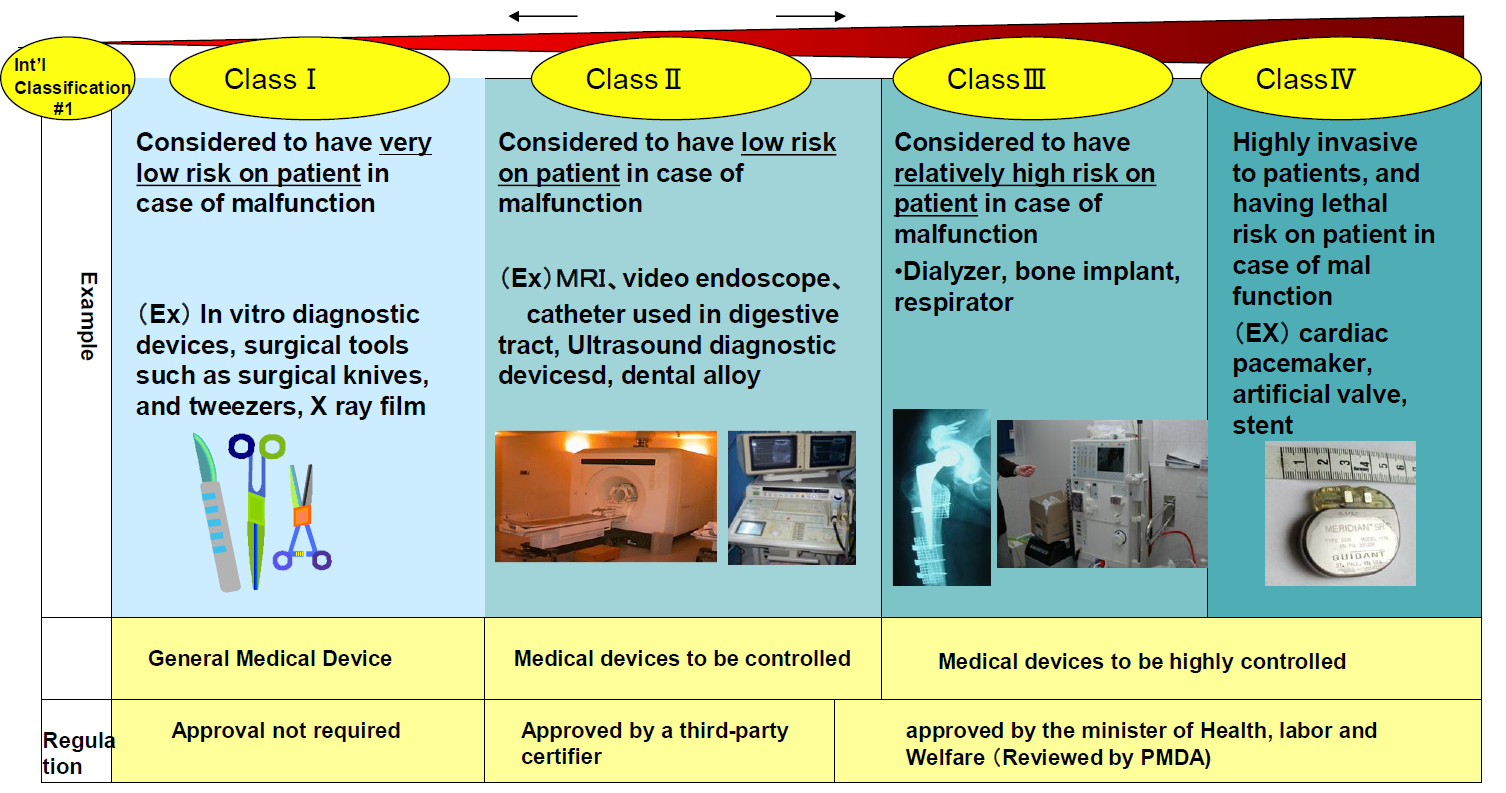
Interoperability standards for medical device integration in the OR and issues relating to international approval procedures (part 4) - ISCASBlog
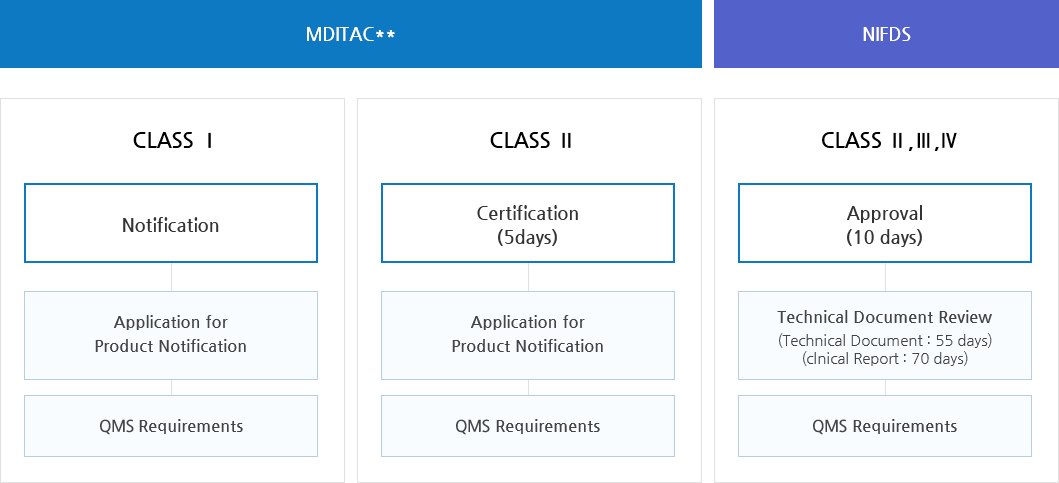
Ministry of Food and Drug Safety>Our Works>Medical Devices>Approval Process
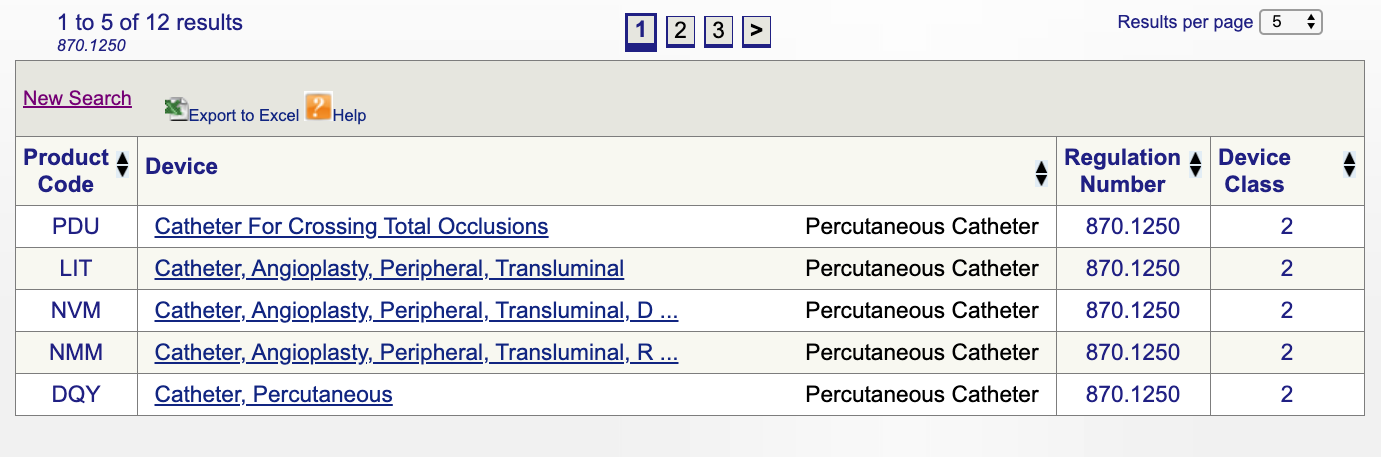
Medical Device Classification Guide - How To Determine Your Device Class

New world order 2013
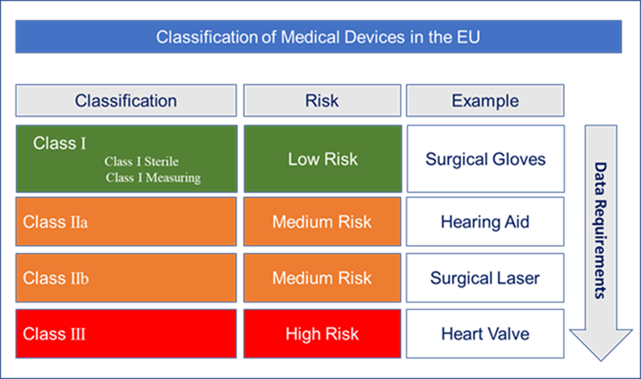
Approaching MDR Compliance
Classify Your Medical Device

General classification and application types of medical devices for

Conformity assessment procedure related to the classification

What's the Difference between a Class I Medical Device and a Class II?
:max_bytes(150000):strip_icc()/Best-Sneakers-for-Wide-Feet-Byrdie-Tout-defacff49936444b98f95af0ffa34d89.jpg)