- Home
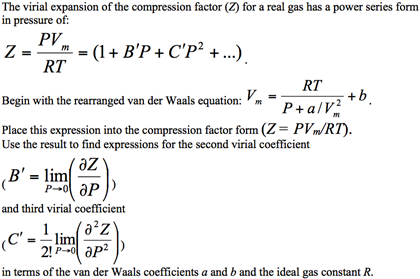
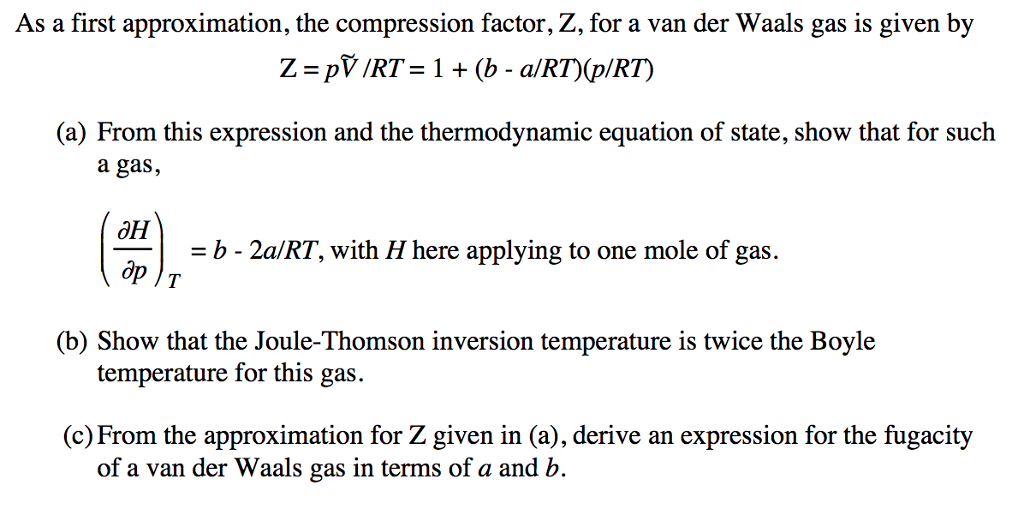
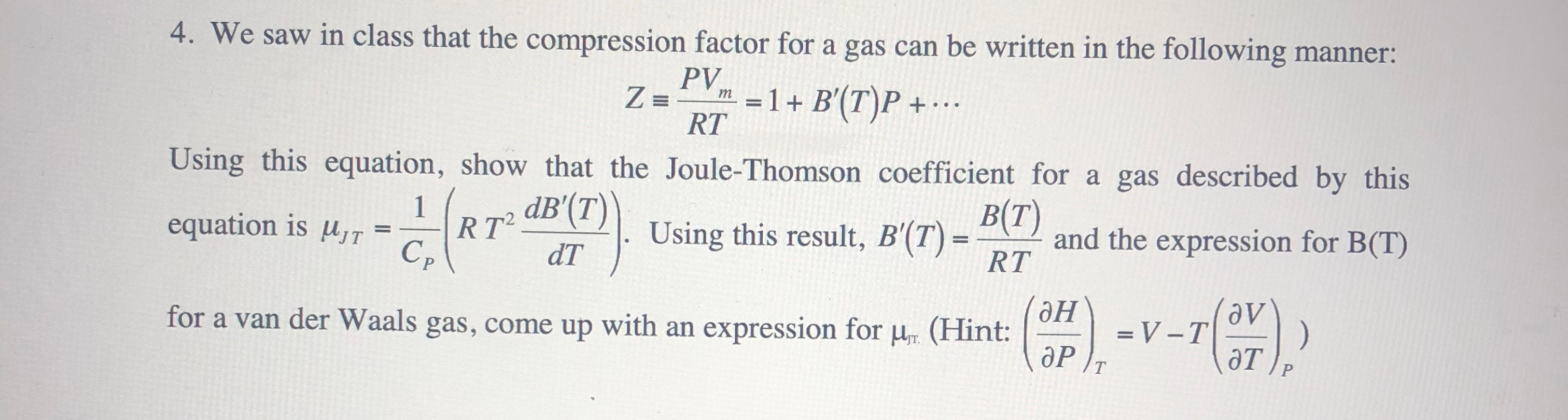
- compression factor equation
- Compression Factor Exam Problem using Molar Volumes - Fully Explained!
Compression Factor Exam Problem using Molar Volumes - Fully Explained!
5 (590) · $ 14.50 · In stock

Physical Chemistry The Compression Factor (Z) [w/1 example]

Determining the Work Done by an Isothermal Process., Chemistry

Polymers, Free Full-Text

Compression Factor Exam Problem using Molar Volumes - Fully Explained!
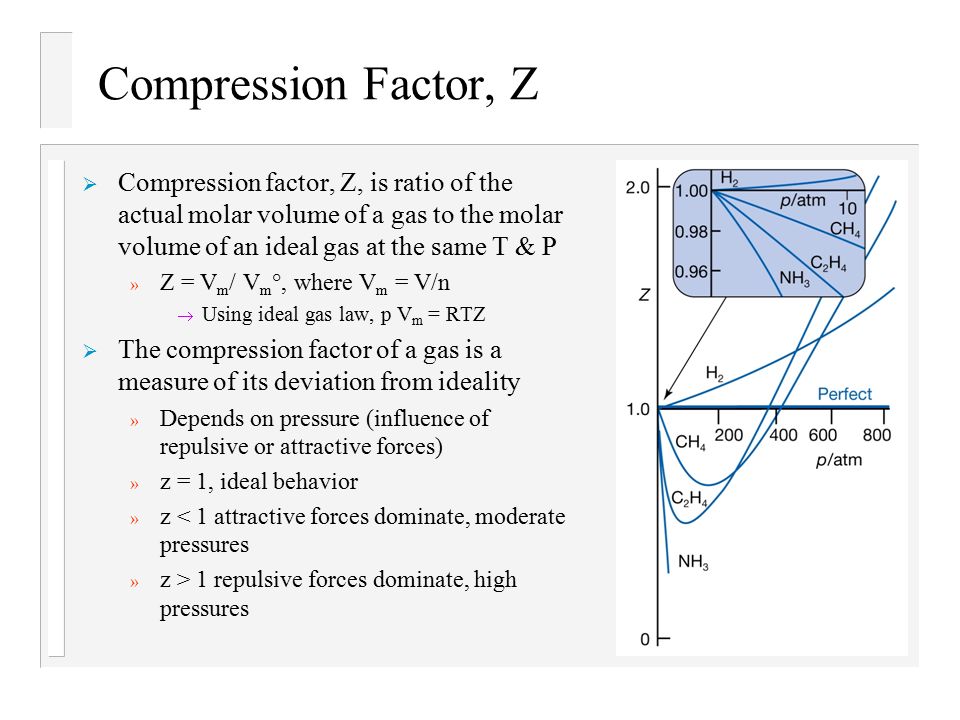
Text: Physical Chemistry, 7th Edition, Peter Atkins and J. de Paula - ppt download

Solved Suppose that 11.3 mol C2H6(g) is confined to 4.860

A phase-field chemo-mechanical model for corrosion-induced cracking in reinforced concrete - ScienceDirect

A gas has a compressibility factor of 0.5 and a molar volume of 0.4 dm3 mol−1 at temperature of 800K

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
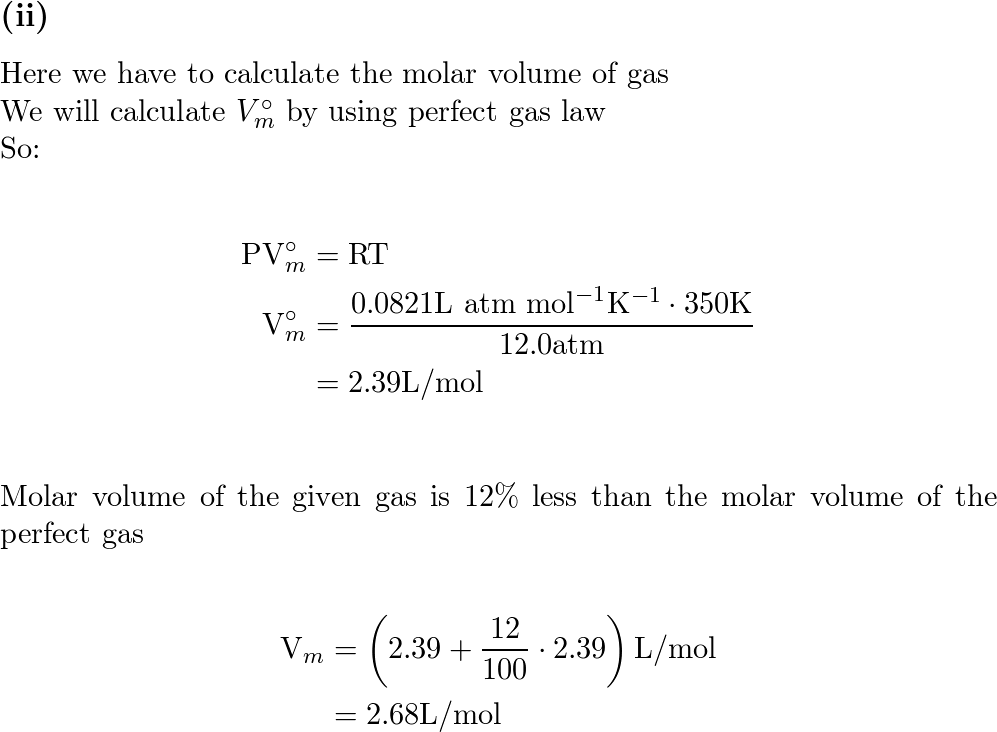
a) A gas at 250 K and 15 atm has a molar volume 12 per cent