Molecules, Free Full-Text
5 (536) · $ 17.00 · In stock
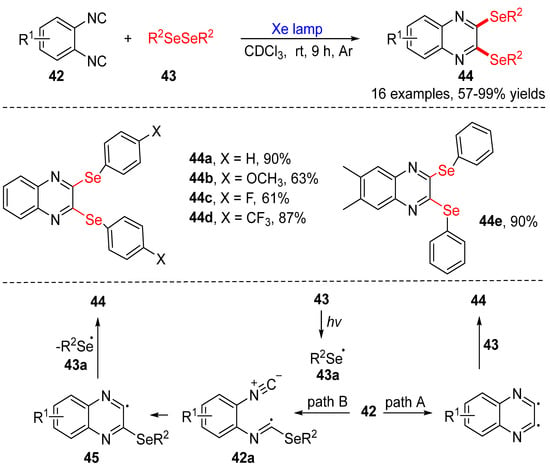
Dichalcogenides (disulfides and diselenides), as reactants for organic transformations, are important and widely used because of their potential to react with nucleophiles, electrophilic reagents, and radical precursors. In recent years, in combination with photochemical technology, the application of dichalcogenides as stable radical reagents has opened up a new route to the synthesis of various sulfur- and selenium-containing compounds. In this paper, synthetic strategies for disulfides and diselenides and their applications with photochemical technology are reviewed: (i) Cyclization of dichalcogenides with alkenes and alkynes; (ii) direct selenylation/sulfuration of C−H/C−C/C−N bonds; (iii) visible-light-enabled seleno- and sulfur-bifunctionalization of alkenes/alkynes; and (iv) Direct construction of the C(sp)–S bond. In addition, the scopes, limitations, and mechanisms of some reactions are also described.

Molecules, Free Full-Text, onoo 2
Molecules, Free Full-Text, mate pastor con negras
Molecules, Free Full-Text, mate pastor con negras
Molecules, Free Full-Text
Molecules, Free Full-Text, tadi & imperio1979
Molecules Free Full-Text Research And Development Of, 59% OFF
Molecules, Free Full-Text, mdpope 3
Molecules Free Full Text The Role Of Catechins In Cellular

Molecules, Free Full-Text, chinese wall cpa 20
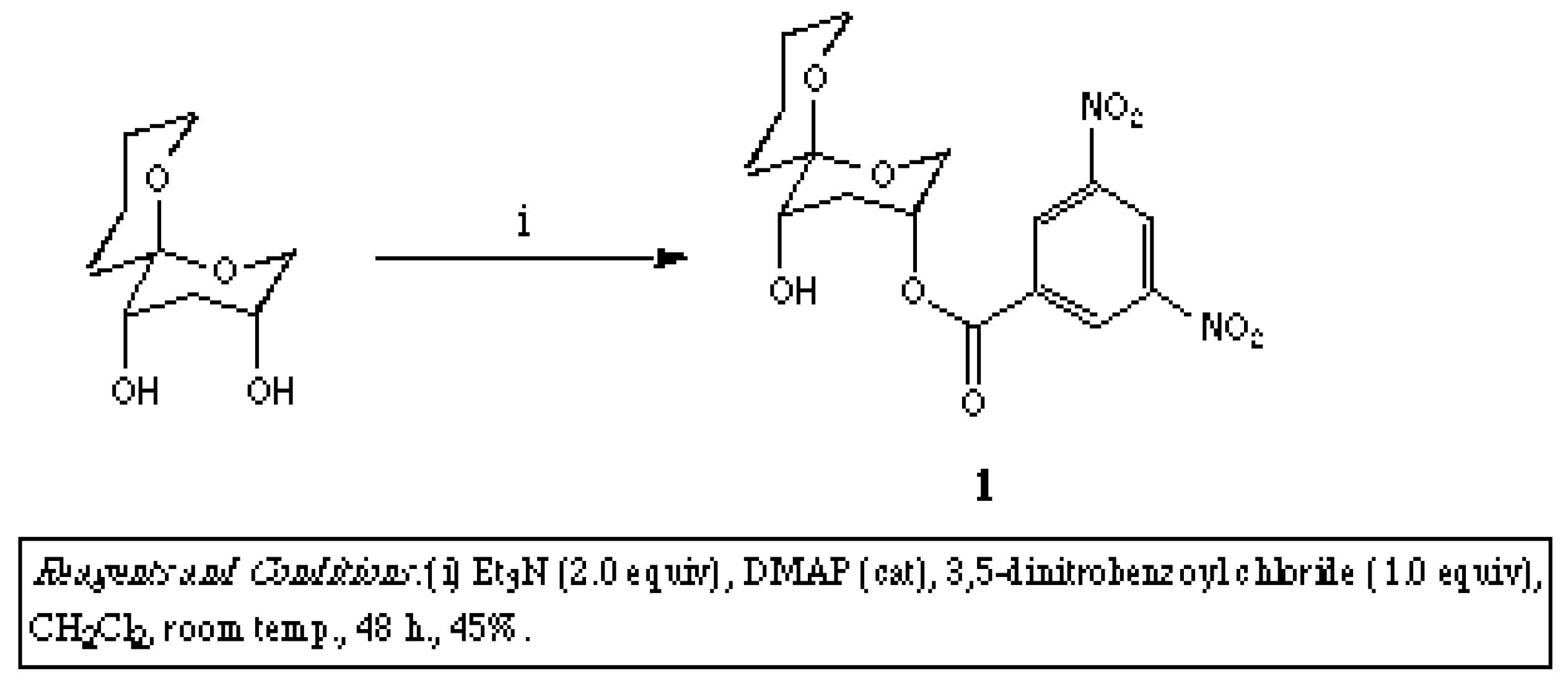
Molecules, Free Full-Text
Molecules, Free Full-Text, Stearic Acid

Molecules, Free Full-Text, kurnik dominó
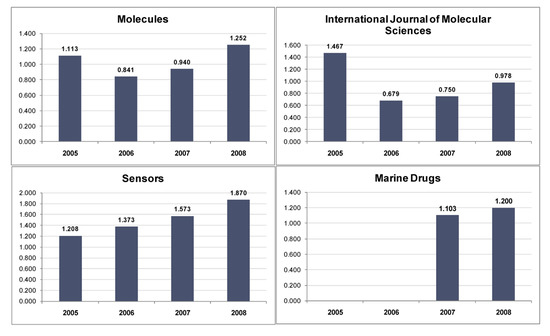
Molecules, Free Full-Text, csvp portal

Molecules An Open Access Journal from MDPI