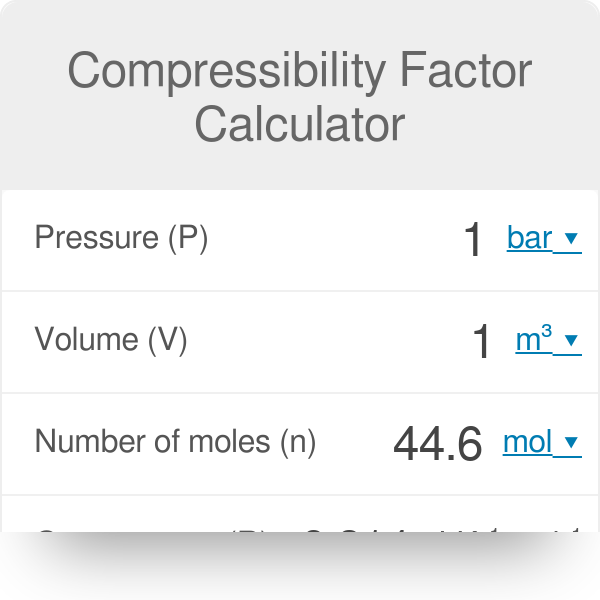
Solved RT B 2. The compressiblity factor for a gas is
4.5 (333) · $ 9.99 · In stock
Answer to Solved RT B 2. The compressiblity factor for a gas is

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

Non-Ideal Gas Behavior Chemistry: Atoms First
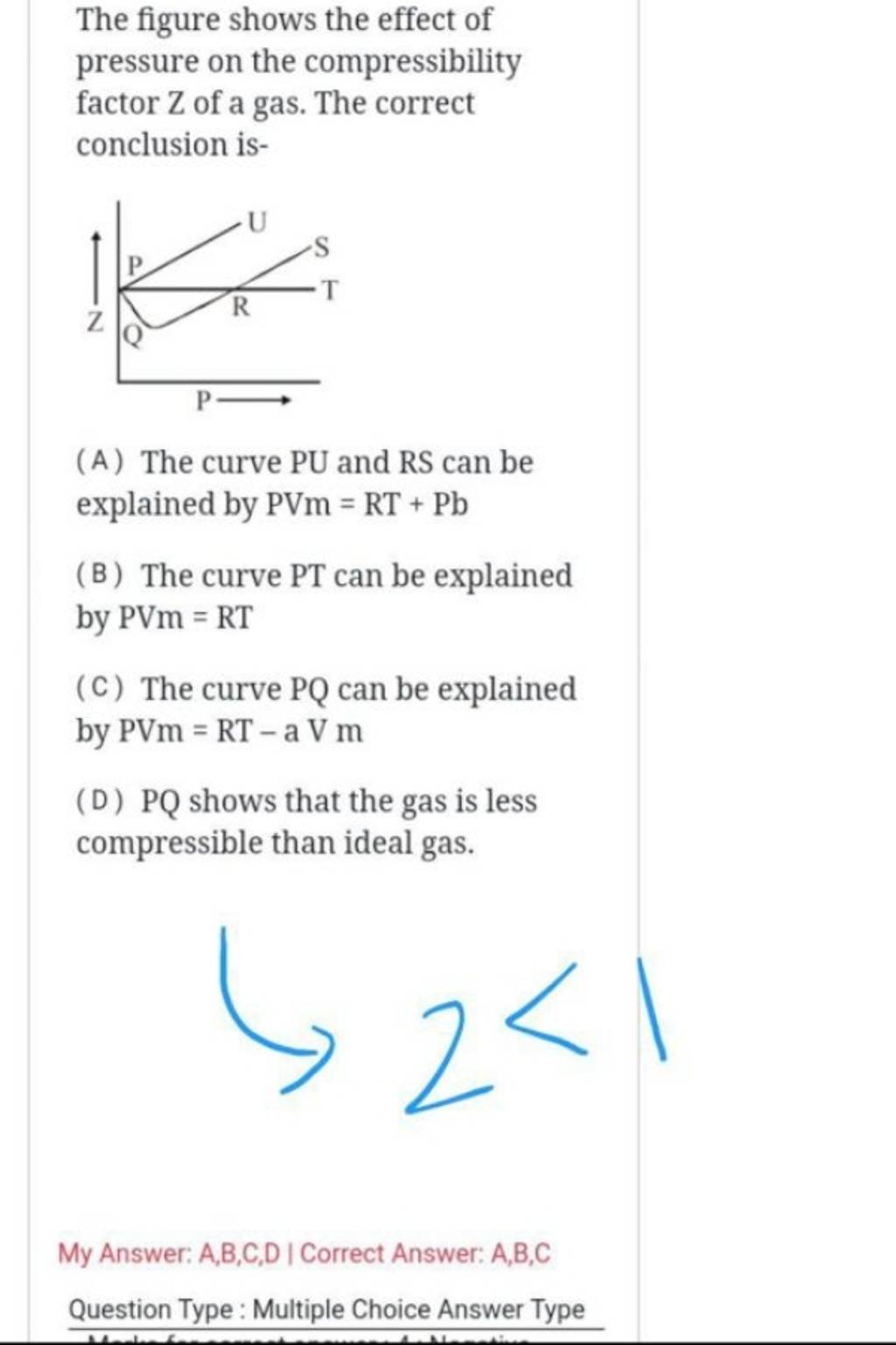
The figure shows the effect of pressure on the compressibility factor Z o..
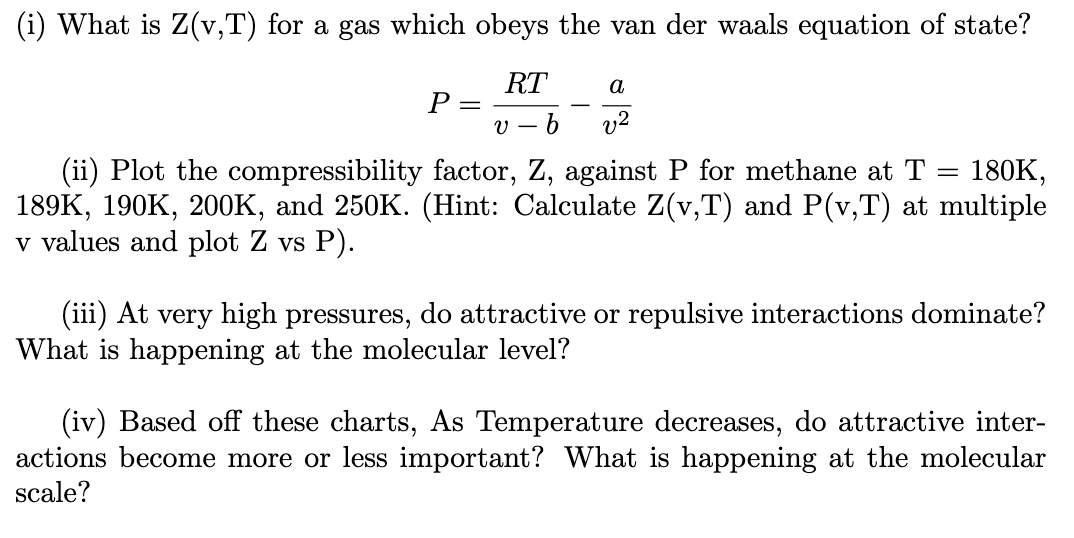
Solved (i) What is Z(v,T) for a gas which obeys the van der
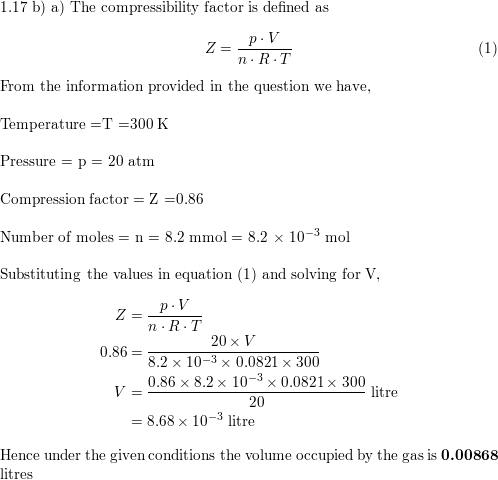

A certain gas obeys P(Vₘ - b) = RT. The value of (∂Z/∂P)ₜ is xb

The compressibility factor for a real gas at high pressure is .

OneClass: 2. Fugacity for a van-der-Waals gas Let's get a feel for how much fugacity deviates from pr

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
How can we calculate critical temperature, volume and pressure in terms of a and b? - Quora