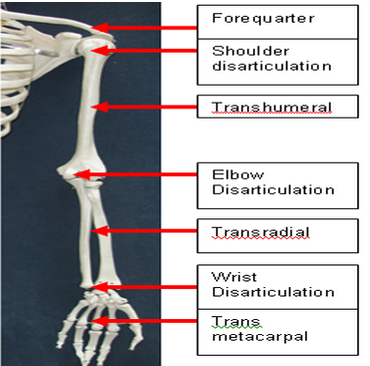
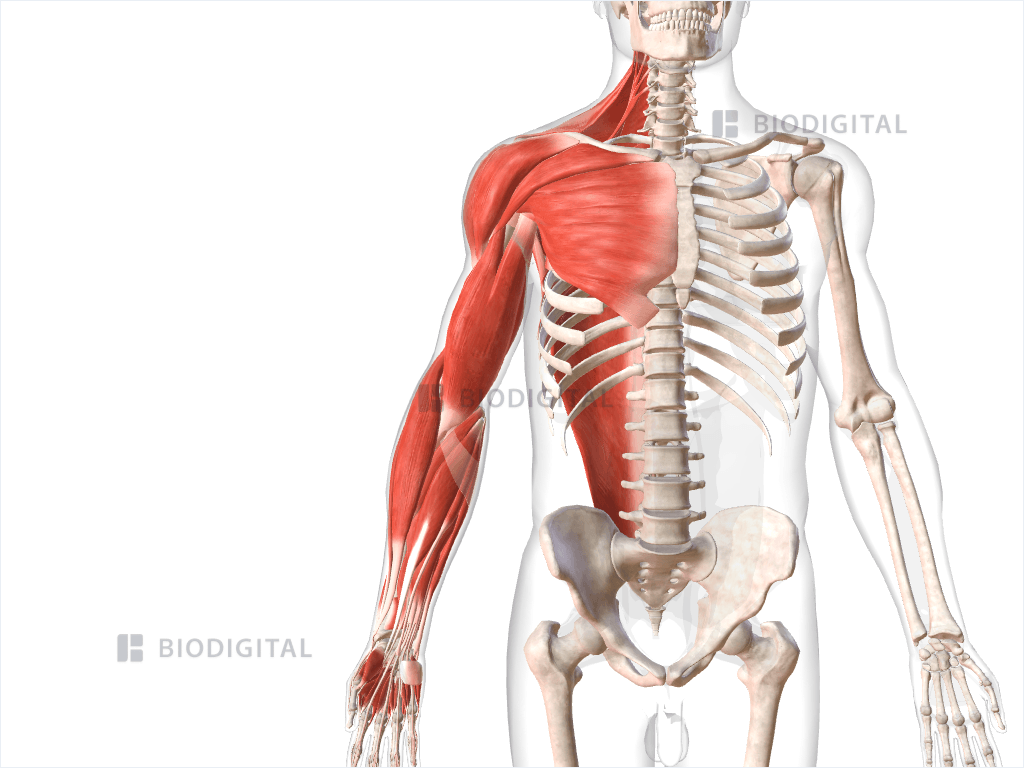
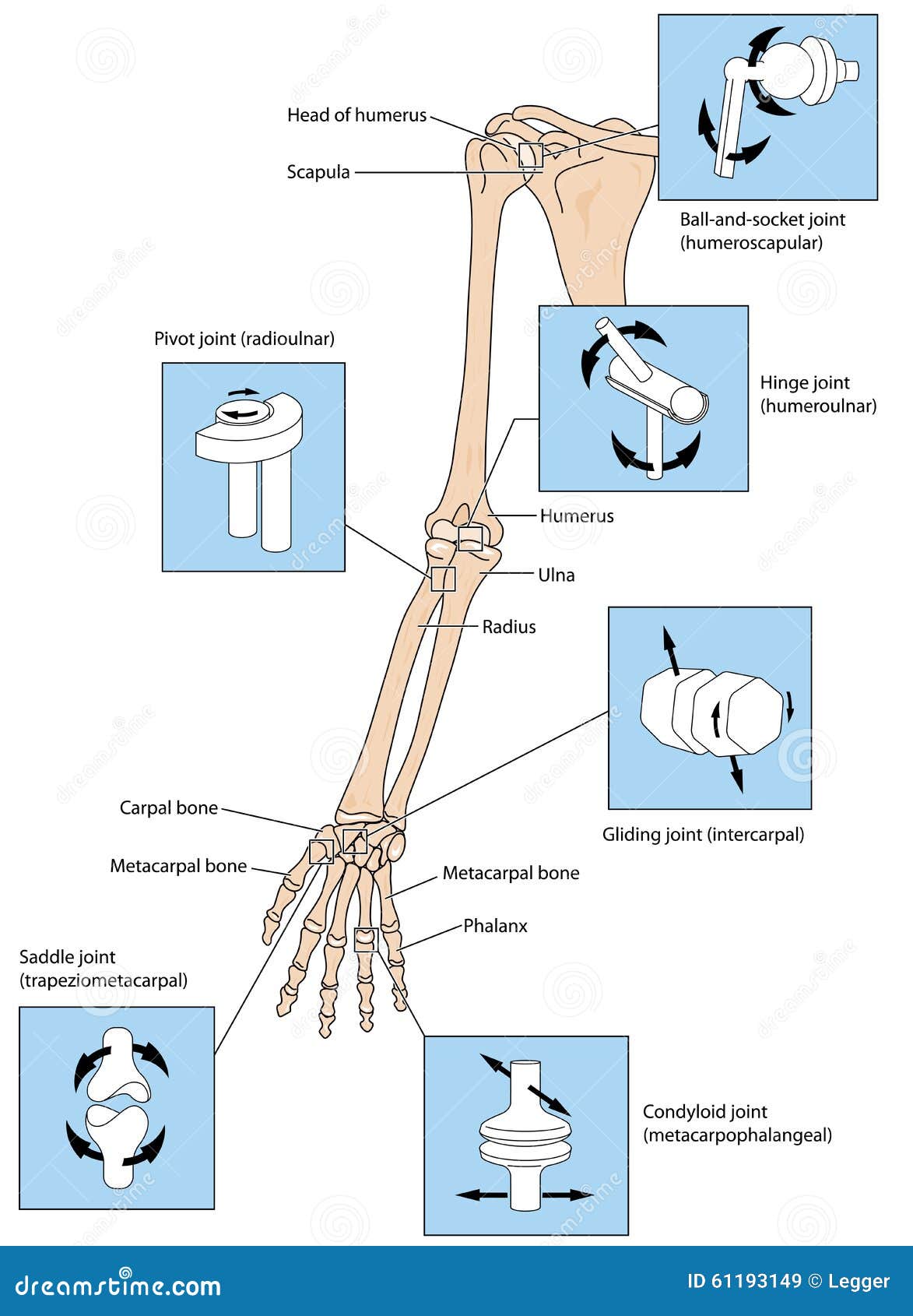
Upper Limb Spasticity - Revance
4.8 (719) · $ 20.99 · In stock

RVNC Stock: How IBD 50's Leader Is Taking On 'Prestige' Medical Aesthetics

Journal of Rehabilitation Medicine - Sustained efficacy of incobotulinumtoxina repeated injections for upper-limb post-stroke spasticity: A post hoc analysis - HTML
Revance Therapeutics Gets Permanent J-Code for Daxxify -February 02, 2024 at 08:33 am EST

OnabotulinumtoxinA sBLAs for Upper, Lower Limb Spasticity Accepted by FDA
Welcome to Texas Neurology

DaxibotulinumtoxinA Aims to Be Next Big Post-Stroke Spasticity Treatment

Revance Reports Positive Results from ASPEN-1 Phase 3 Trial of DaxibotulinumtoxinA for Injection in Cervical Dystonia

PDF) Individualized OnabotulinumtoxinA Treatment for Upper Limb Spasticity Resulted in High Clinician‐ and Patient‐Reported Satisfaction: Long‐Term Observational Results from the ASPIRE Study
Articles about Revance Therapeutics, Inc.

Revance Announces FDA Approval of DAXXIFY™ (DaxibotulinumtoxinA-lanm) for Injection, the First and Only Peptide-Formulated Neuromodulator With Long-Lasting Results

Is Daxi the new Botox?
FDA Serves Omeros, MannKind-United and Revance with CRLs
Upper extremity support OKG-05 Reh4Mat – lower limb orthosis and braces - Manufacturer of modern orthopaedic devices