- Home
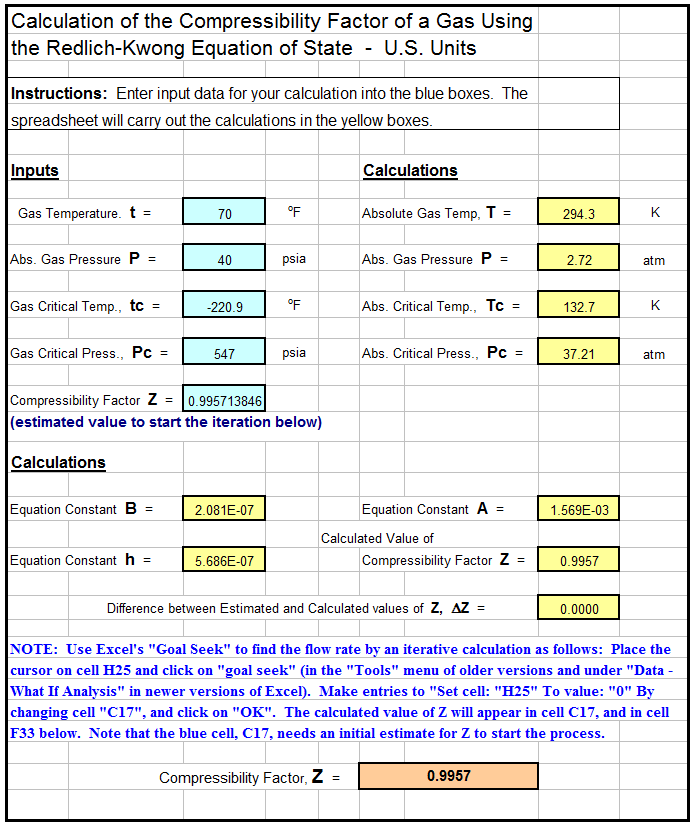
- compressibility factor equation
- Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of
Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of
4.7 (325) · $ 9.00 · In stock

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

1148 questions with answers in GAS

Welcome to Chem Zipper.com: A closed tank has two compartments A and B, both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is

The compression factor (compressibility factor) for 1 mol of a van der
How do Van der Waals constants a and b depend on temperature, pressure and volume? - Quora

Solved APPENDIX Problem 1: Molar Volume and Compressibility

Welcome to Chem Zipper.com: THE STATE OF MATTER

Solved At high gas densities, the van der Waals equation der

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

The compressibility factor for 1 mole of a Vander Waals gas at the Boyle's temperature is - a. 1+


1148 questions with answers in GAS

I need help with question 3: a,b,c, i'm stuck and
Université de Genève - Groupe du Professeur Andreas Hauser