physical chemistry - Why do some gases have lower value of Z for a
4.5 (582) · $ 24.50 · In stock
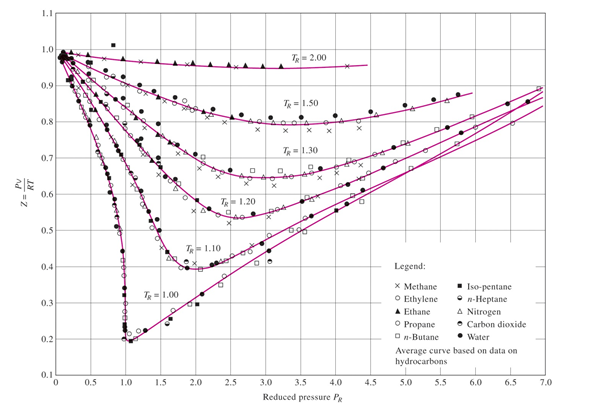
In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
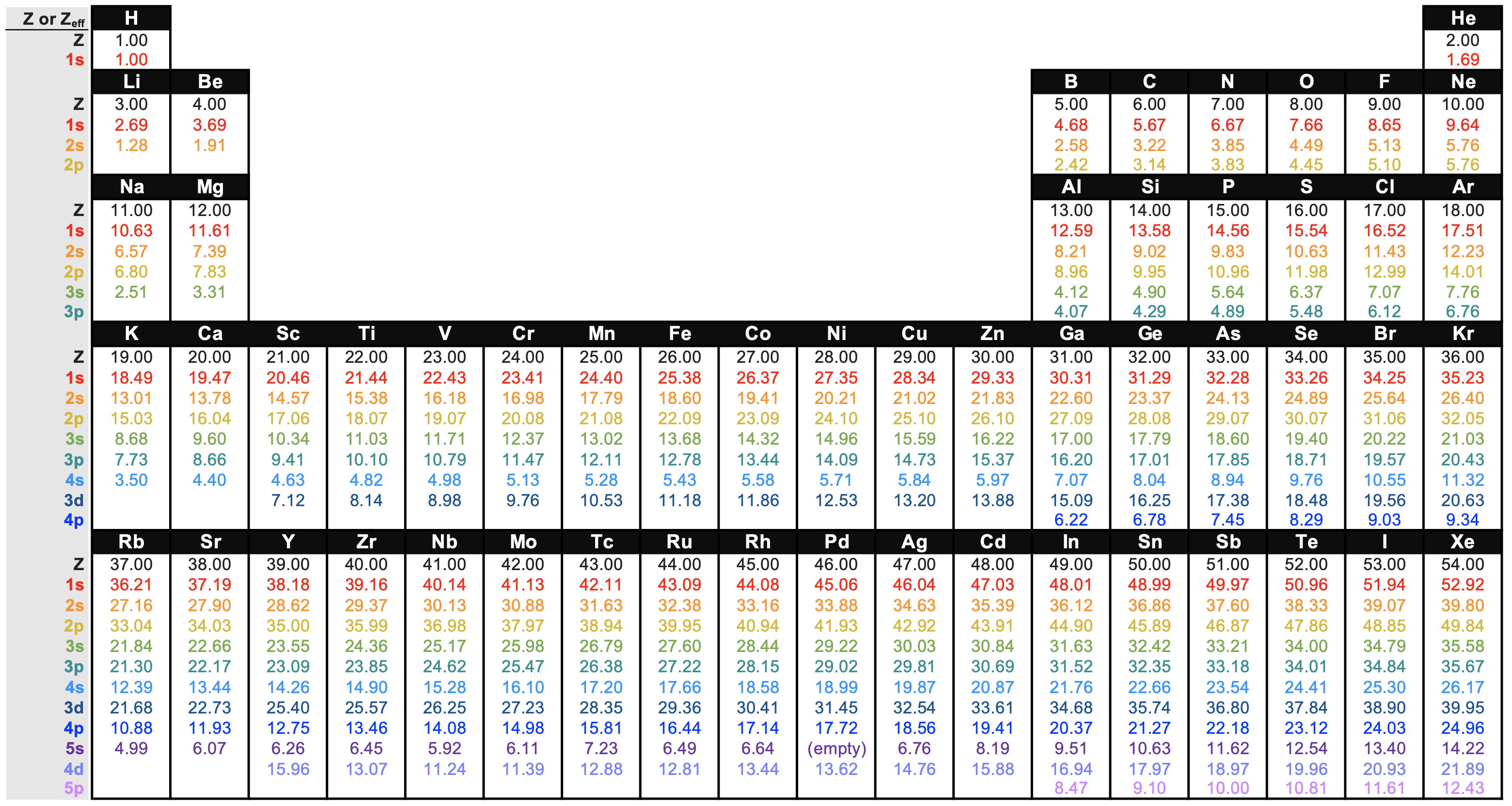
1.1.2: Effective Nuclear Charge - Chemistry LibreTexts

The Ideal Gas Law

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility Factor of Gas Overview, Equation & Chart

Catalysis Chemistry, Classification, & Chemical Reactions

Write a short note on liquefaction of gases. from Chemistry Sta

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Non-Ideal Gas Behavior Chemistry: Atoms First

Gas (Gaseous State) - Characteristics, Properties, Video, FAQs