200 g of a sample of limestone liberates 66 g of CO2 on heating
4.7 (401) · $ 9.99 · In stock
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

Topical Mock Chemistry Questions, PDF, Chemical Elements

Punjabi] When 200g of lime stone is strongly heated, it undergoes the

A review on chemical precipitation in carbon capture, utilization and storage, Sustainable Environment Research

Cement testing
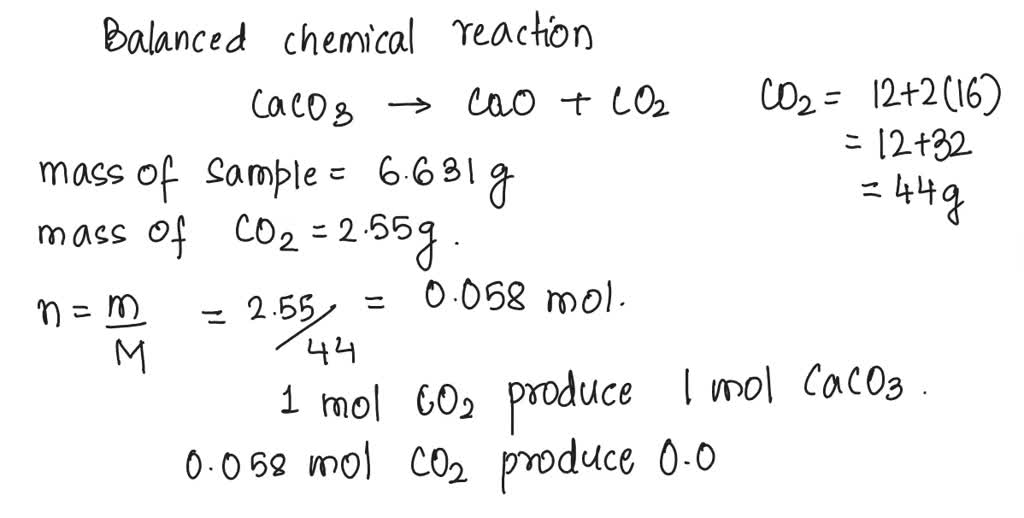
SOLVED: A sample of limestone and other soil materials was heated, and the limestone decomposed to give calcium oxide and carbon dioxide. CaCO3 (s) â†' 3 CaO(s) + CO2 (g) A 6.631

PDF) Quimica Analitica Hamilton
When a limestone of mass 150g was heated until it decomposed to CaO, only 63g of CaO were obtained. What is the percentage purity of the limestone? - Quora
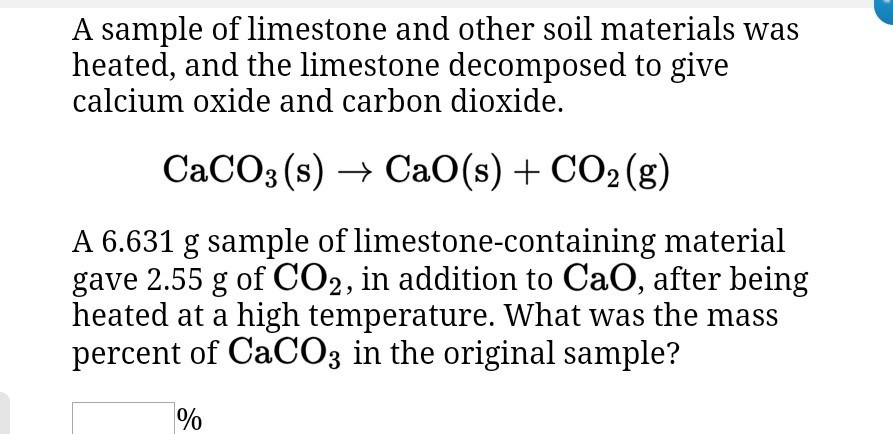
Solved A sample of limestone and other soil materials was
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95

400 g sample of limestone liberates 44 g carbon dioxide on heating. The p..

2 CHEMISTRY , PART-1_490-896 - Flipbook by santanu.bej

PDF) Sequestration of Carbon Dioxide in Coal with Enhanced Coalbed Methane RecoveryA Review †











