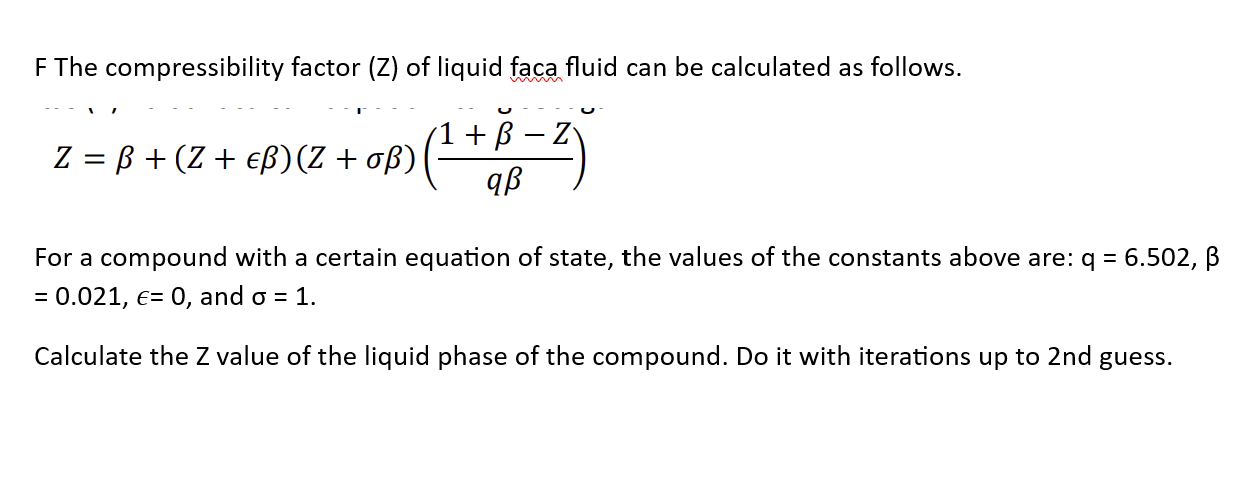
OneClass: For a real gas, the compressibility factor, Z, is
5 (198) · $ 18.50 · In stock

OneClass: At low pressures the compressibility factor for a Van der Waal's gas is given by Z-1+[b- (a

Compressibility factor Z - Gaseous State

Solved The compression factor (Z) for a real gas can be

Solved The compressibility factor, Z, can be thought of as a

Description of real gases: Compression factor

Compressibility factor (z): real gases deviate from ideal behav-Turito

If z<1, does it mean that the gases behave more like perfect or real gases? - Quora

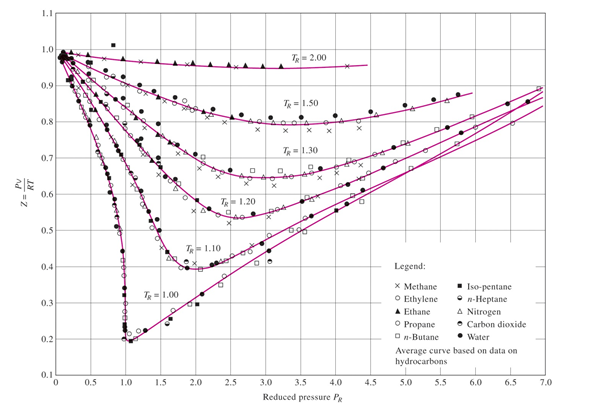
Compressibility factor - Wikipedia

Compressibility factor - Wikipedia
If z<1, does it mean that the gases behave more like perfect or real gases? - Quora

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Compressibility factor - Wikipedia
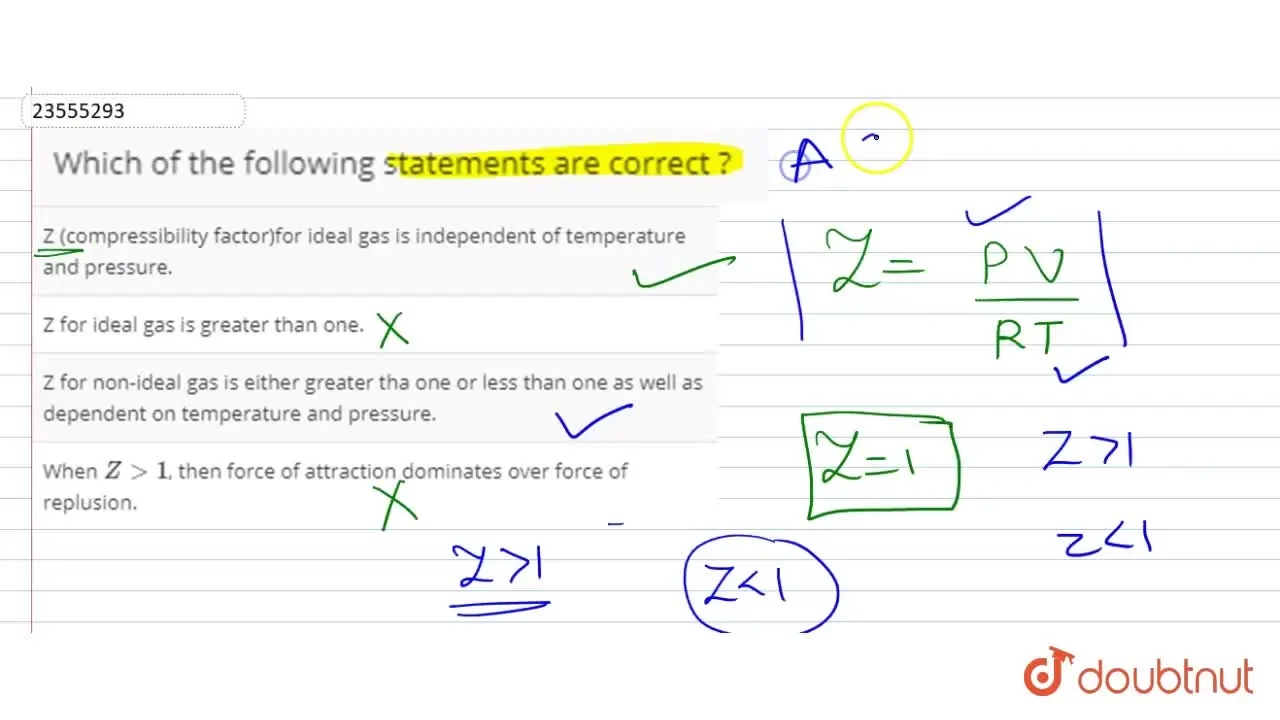
Z for non-ideal gas is either greater tha one or less than one as well

Compressibility factor for real gases