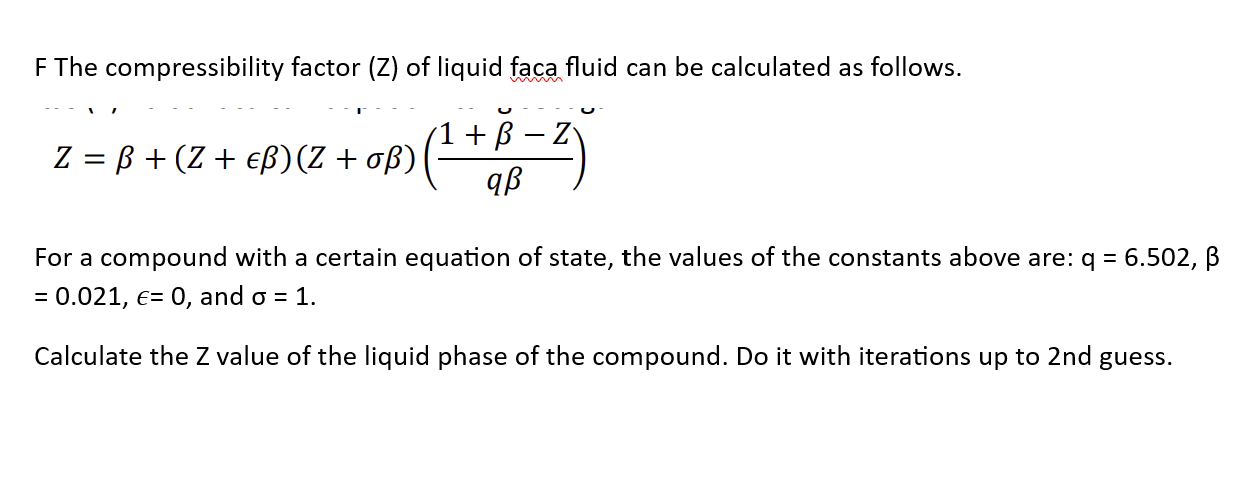
For H(2) gas, the compressibility factor,Z = PV //n RT is
4.5 (600) · $ 13.00 · In stock
For H(2) gas, the compressibility factor,Z = PV //n RT is
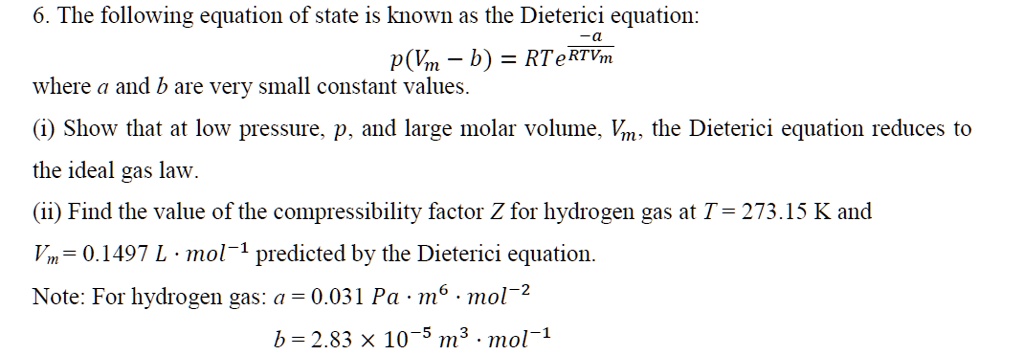
SOLVED: The following equation of state is known as the Dieterici equation: p(Vm - b) = RT * e^(RT/Vm), where a and b are very small constant values. Show that at low

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced
Under what conditions do you expect a real gas such as hydrogen gas to behave like an ideal gas? - Quora

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Compressibility factor of n-decane vapor (upper graph) and of ethylene
The given graph represent the variations of compressibility factor (z) = pV/ nRT versus p, - Sarthaks eConnect
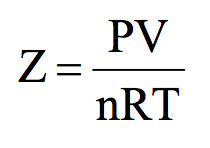
Real Gases - Chemistry, Class 11, States of Matter
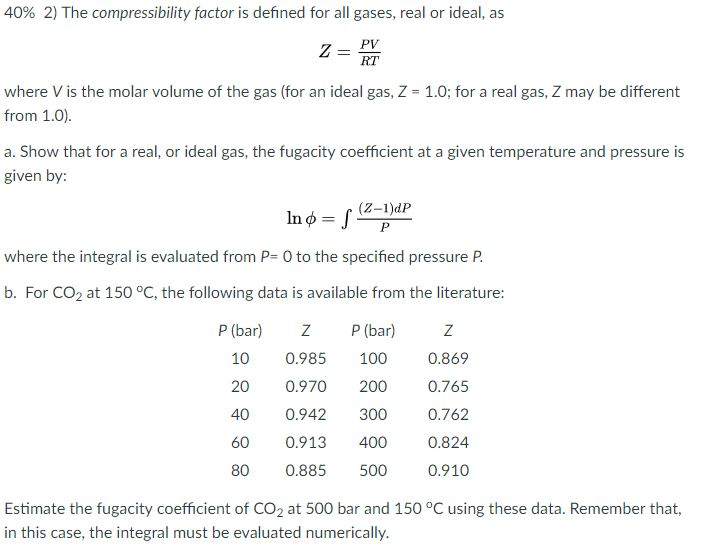
Solved 40% 2) The compressibility factor is defined for all

6.3: Van der Waals and Other Gases - Physics LibreTexts

85. For H, gas, the compressibility factor, Z=PV/n RT is : (a) equal to 1 (b) equal to 0 (c) always greater than 1 (d) initially than 1 and then becomes greater than high pressures

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

e Compressibility factor (Z) for hydrogen WRT pressure and temperature