Protocol for a randomised controlled feasibility trial of exercise
4.6 (738) · $ 30.99 · In stock
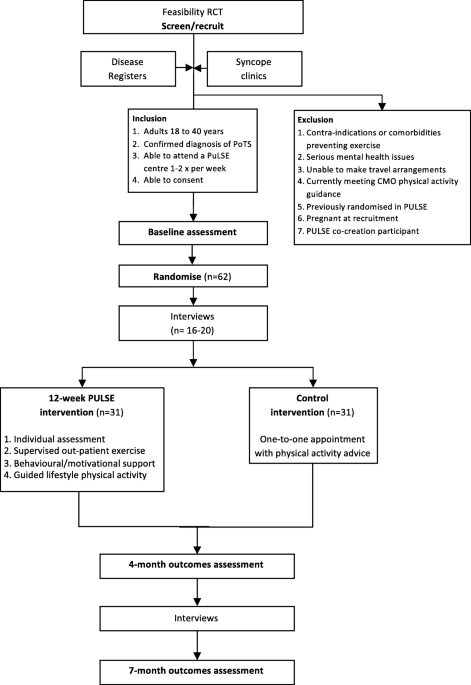
Background Postural orthostatic tachycardia syndrome (POTS) is an autonomic nervous system disorder causing an abnormal cardiovascular response to upright posture. It affects around 0.2% of the population, most commonly women aged 13 to 50 years. POTS can be debilitating; prolonged episodes of pre-syncope and fatigue can severely affect activities of daily living and health-related quality of life (HRQoL). Medical treatment is limited and not supported by randomised controlled trial (RCT) evidence. Lifestyle interventions are first-line treatment, including increased fluid and salt intake, compression tights and isometric counter-pressure manoeuvres to prevent fainting. Observational studies and small RCTs suggest exercise training may improve symptoms and HRQoL in POTS, but evidence quality is low. Methods Sixty-two people (aged 18–40 years) with a confirmed diagnosis of POTS will be invited to enrol on a feasibility RCT with embedded qualitative study. The primary outcome will be feasibility; process-related measures will include the number of people eligible, recruited, randomised and withdrawn, along with indicators of exercise programme adherence and acceptability. Secondary physiological, clinical and health-related outcomes including sub-maximal recumbent bike exercise test, active stand test and HRQoL will be measured at 4 and 7 months post-randomisation by researchers blinded to treatment allocation. The PostUraL tachycardia Syndrome Exercise (PULSE) intervention consists of (1) individual assessment; (2) 12-week, once to twice-weekly, supervised out-patient exercise training; (3) behavioural and motivational support; and (4) guided lifestyle physical activity. The control intervention will be best-practice usual care with a single 30-min, one-to-one practitioner appointment, and general advice on safe and effective physical activity. For the embedded qualitative study, participants (n = 10 intervention, n = 10 control) will be interviewed at baseline and 4 months post-randomisation to assess acceptability and the feasibility of progressing to a definitive trial. Discussion There is very little high-quality research investigating exercise rehabilitation for people with POTS. The PULSE study will be the first randomised trial to assess the feasibility of conducting a definitive multicentre RCT testing supervised exercise rehabilitation with behavioural and motivational support, compared to best-practice usual care, for people with POTS. Trial registration ISRCTN45323485 registered on 7 April 2020.

A study protocol for a randomised controlled feasibility trial of an intervention to increase activity and reduce sedentary behaviour in people with severe mental illness: Walking fOR Health (WORtH) Study

Sensors, Free Full-Text
journal-article.927aefe.svg

Exercise Guidelines for Postural Tachycardia Syndrome
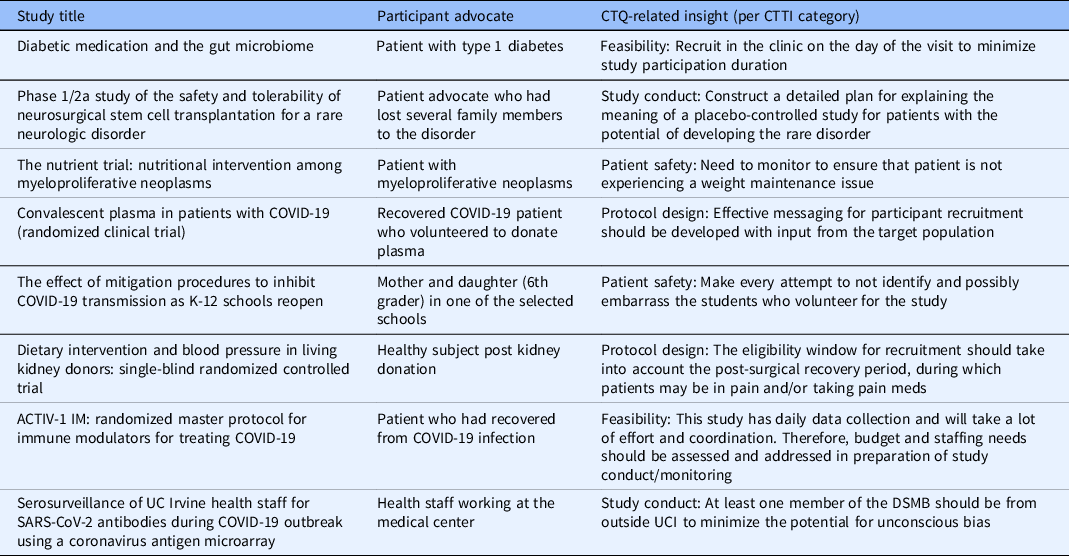
Feasibility and acceptability of a structured quality by design approach to enhancing the rigor of clinical studies at an academic health center, Journal of Clinical and Translational Science

Sandeep PANIKKER, Honorary Consultant Cardiologist and Electrophysiologist, Doctor of Philosophy, Imperial College London, London, Imperial, National Heart and Lung Institute

Pilot and Feasibility Studies

Helen EFTEKHARI, Arrhythmia & Syncope Advanced Nurse Practitioner, RGN, BSC (Hons) Nursing, MSC Advancing Nurse Practice (MSC). NIHR MRES, University Hospitals Coventry and Warwickshire NHS Trust, Coventry
Defining Feasibility and Pilot Studies in Preparation for Randomised Controlled Trials: Development of a Conceptual Framework

PDF) Protocol update for a randomised controlled feasibility trial of exercise rehabilitation for people with postural tachycardia syndrome: the PULSE study
A randomised controlled feasibility trial for an educational school-based mental health intervention: study protocol

PDF) Protocol update for a randomised controlled feasibility trial of exercise rehabilitation for people with postural tachycardia syndrome: the PULSE study