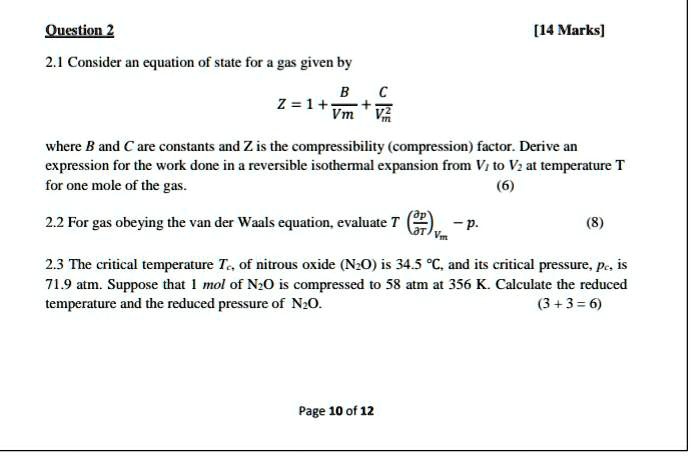
Derive an expression for the compression factor of a gas tha
4.5 (240) · $ 12.00 · In stock

Physical Chemistry The Compression Factor (Z) [w/1 example]
Energies, Free Full-Text
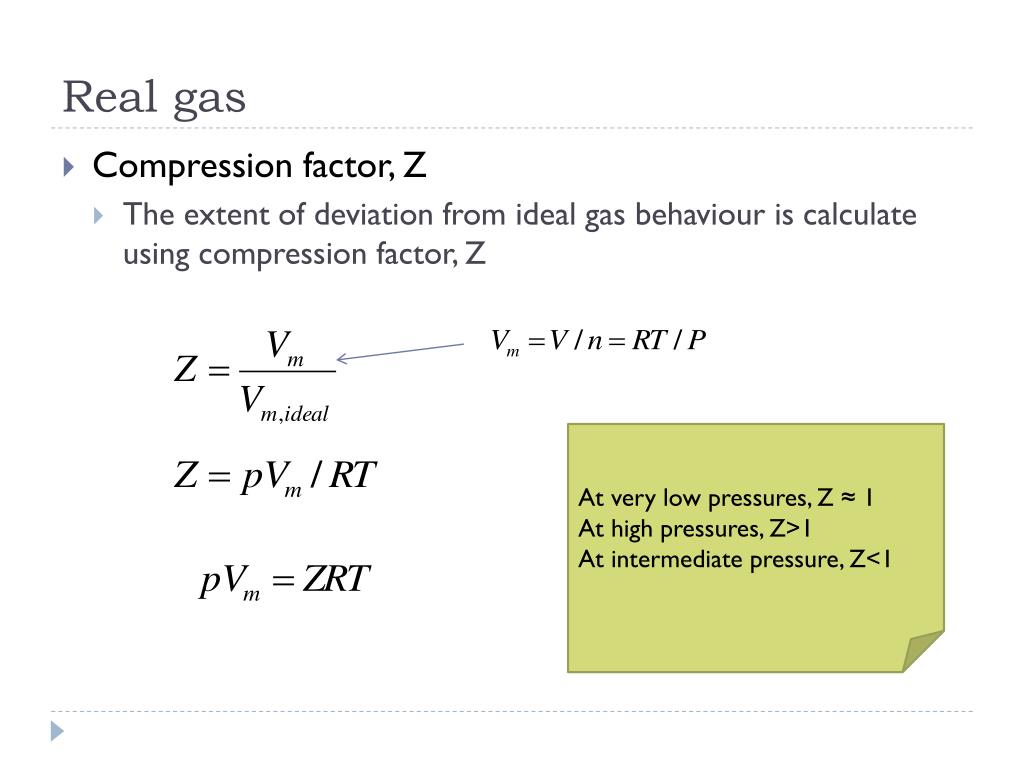
PPT - ERT 108 Physical Chemistry INTRODUCTION-Part 2 PowerPoint Presentation - ID:2630974

Solved (b) Hydrogen was collected by downward displacement

Atkins' Physical Chemistry [11 ed.] 9780191082559

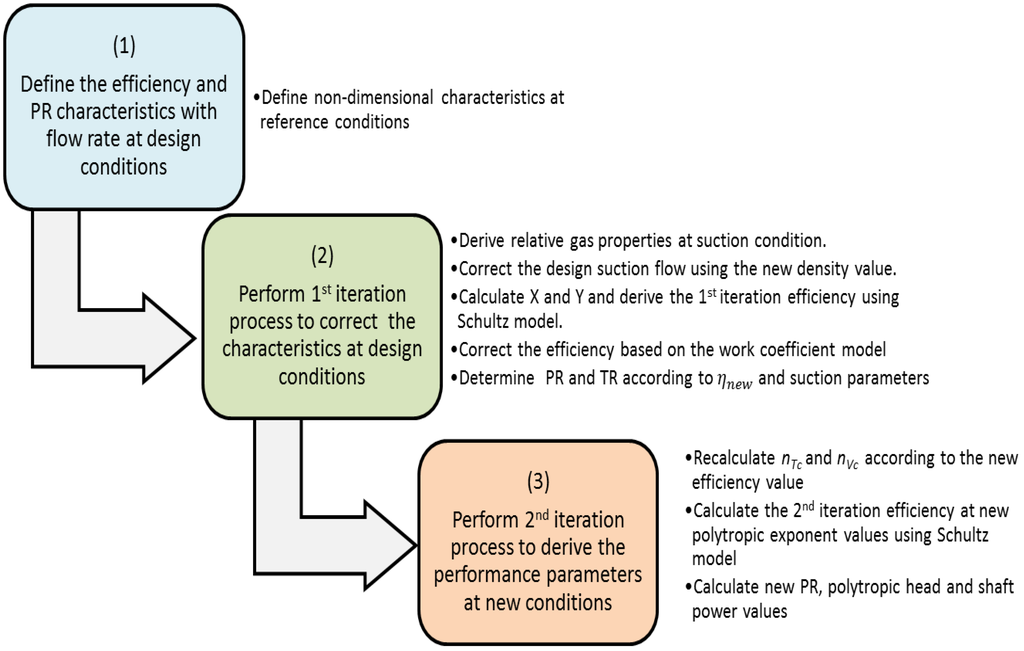
Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

OneClass: Derive an expression for the compression factor of a gas thatobeys the equation of state p(

Real Gas Behavior The Compression Factor (Z) [Example #2]
The value of compression factor at the critical state of a vander waals gas is

The formula compressibility of a gas is- z=dfrac{pv}{nRT}dfrac{1}{P}dfrac{dv}{dp} V. dfrac{dP}{dV}dfrac{1}{V}.dfrac{dV}{dP}

Derived properties for engineering applications

1. A gas at 250 K and I atn has a molar volume 5%

SOLVED: The Berthelot equation of state is given by p = V - nbTvZ. The total differential of the compression factor is given by dZ = MdT + Ndv. (a) Write the

Solved 3. Derive an expression for the compression factor of