- Home
- compressibility factor equation
- What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
4.6 (762) · $ 6.50 · In stock
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a ' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}

16.4: The Law of Corresponding States - Chemistry LibreTexts

Compressibility Factor Z Important Concepts and Tips for JEE Main

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
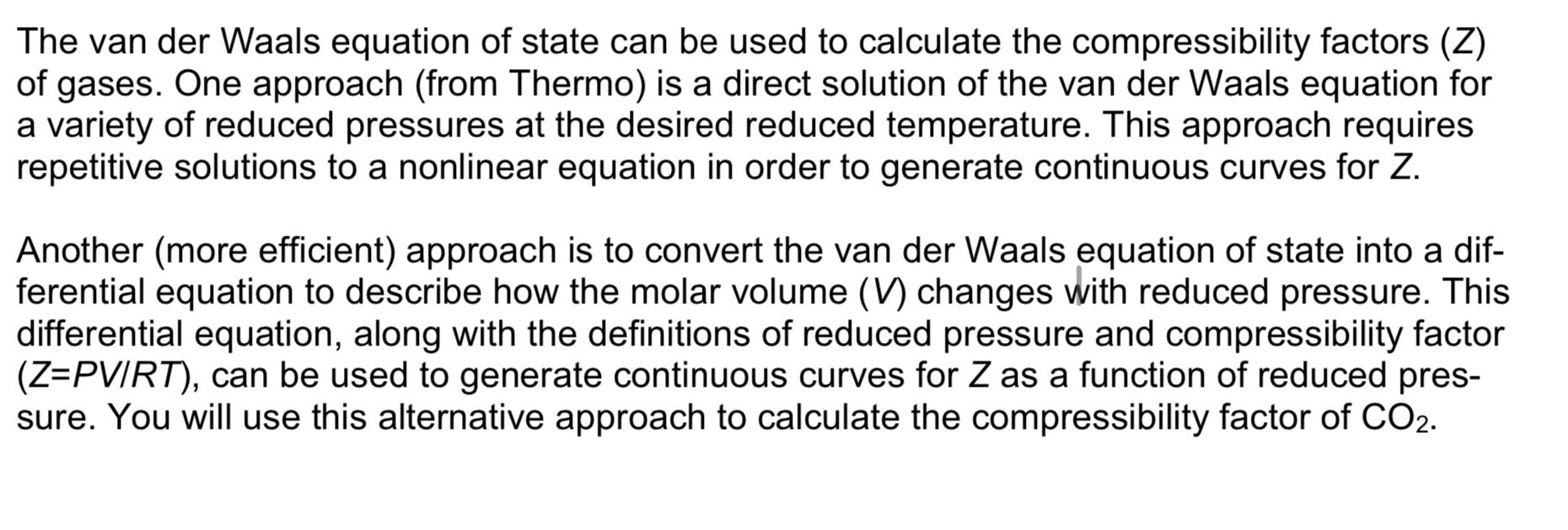
Solved The van der Waals equation of state can be used to
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
Gases, Free Full-Text

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1

Non-Ideal Gas Behavior Chemistry: Atoms First
How I find the a and b constant in the Van der Waals equation? - Quora
Gas Laws - Overview - Chemistry LibreTexts

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application
How I find the a and b constant in the Van der Waals equation? - Quora

NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 - States of Matter: Gases and Liquids




/product/72/354071/2.jpg?5955)




