- Home
- 32 g
- 32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
4.6 (598) · $ 26.99 · In stock
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

103 questions with answers in HYDRAZINE
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting reagent ii)Max amount of H2O formed iii) Amount of reactants which remains unreacted.
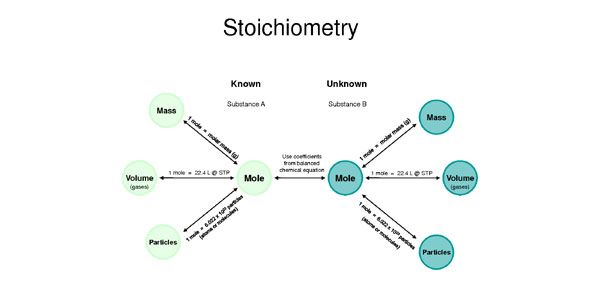
Stoichiometry And Limiting Reagent Review - Quiz, Trivia & Questions

Ethylene oxide - Wikipedia
PPT - Mass Relationships in Chemical Reactions PowerPoint Presentation - ID:3181342
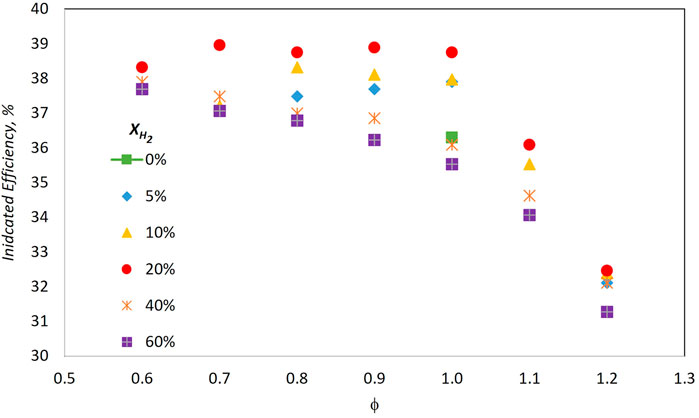
Frontiers Ammonia as Green Fuel in Internal Combustion Engines: State-of-the-Art and Future Perspectives

80 g of `H_(2)` is reacted with 80 g of `O_(2)` to form water. Find out the mass of

Hint : N2(g)+3H2(g)⟶2NH3(g) 28. 80 g of H2 is reacted with 80 g of O2..

GC 1 Flashcards

Aqueous Transformation of a Metal Diformate to a Metal Dihydride Carbonyl Complex Accompanied by H2 Evolution from the Formato Ligands

Review of the Decomposition of Ammonia to Generate Hydrogen

How many grams of water are produced if we react 3 moles of hydrogen with 3 moles of oxygen? About 60 grams

Materials, Free Full-Text
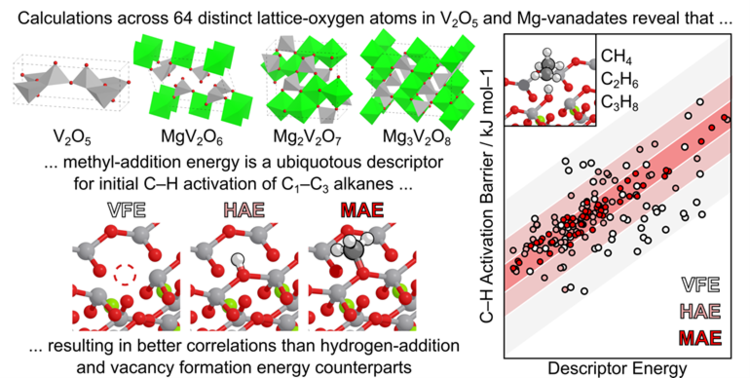
Hibbitts Group Publications
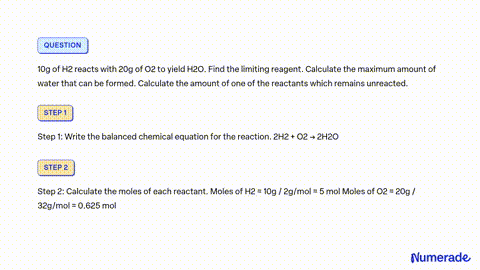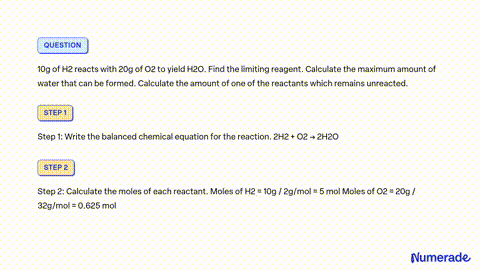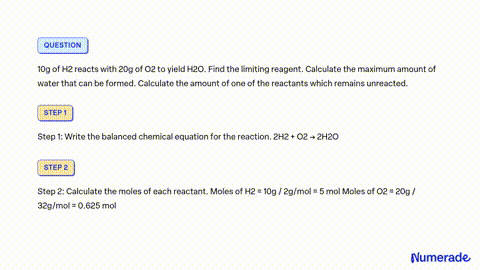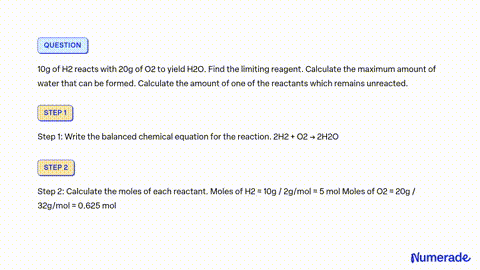
SOLVED: 80 g of H2 is reacted with 80 g of O2 to form water. Find out the mass of water obtained. Which substance is the limiting reagent?










