- Home
- difference between d and dd
- SOLVED: [MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2.
SOLVED: [MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2.
4.6 (320) · $ 22.00 · In stock
VIDEO ANSWER: We can say permanganate permanganate iron, which is here, or we can say intense, intense purple colorati. The oxidation state of the manganese can be found in the m n, o 4 negative. Here, we can say that it is plus 7. The electrons are
[MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2. d-d transitions in the Mn compound compared to the Re compound 3. charge transfer from O to Re compared to O to Mn 4. charge transfer from O to Mn compared to O to Re.
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

PPT - Chemistry 481(01) Spring 2014 PowerPoint Presentation, free download - ID:2249362

Solved (a) Why the [Mn(H20)]2+ complex gives pale pink color

Recent progress in separation of technetium-99 from spent nuclear fuel and radioactive waste. Challenges and prospects - ScienceDirect

PDF) Multi-Centered Solid-Phase Quasi-Intramolecular Redox Reactions of [(Chlorido)Pentaamminecobalt(III)] Permanganate—An Easy Route to Prepare Phase Pure CoMn2O4 Spinel
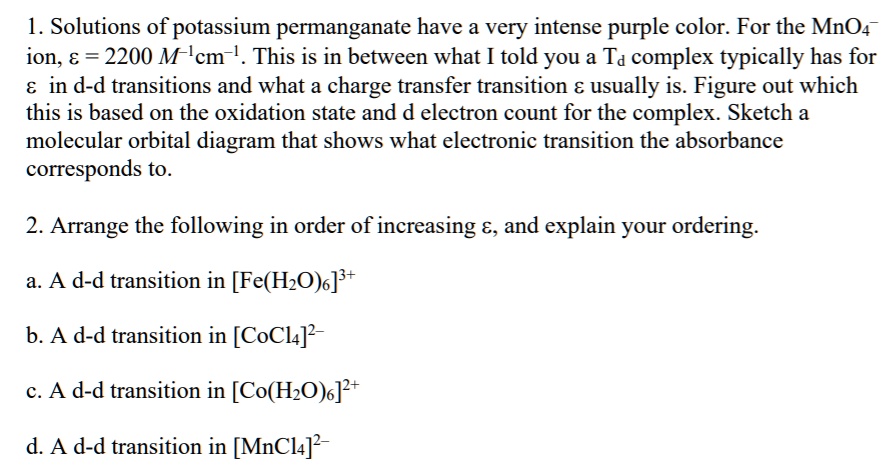
SOLVED: Solutions of potassium permanganate have a very intense purple color. For the MnO4 ion, € = 2200 M-1cm-1. This is in between what I told you a Td complex typically has

ReasonColour of KMnO_4 is due to charge transfer.AssertionKMnO_4 is a colourless compound.

PDF) Multi-Centered Solid-Phase Quasi-Intramolecular Redox Reactions of [(Chlorido)Pentaamminecobalt(III)] Permanganate—An Easy Route to Prepare Phase Pure CoMn2O4 Spinel

coordination compounds - How can the intense color of potassium permanganate be explained with molecular orbital theory? - Chemistry Stack Exchange

8.1.6.4 Sodalite, cancrinite, and leifite groups of silicates

PDF) Applications of Supramolecular Anion Recognition
3.1 Transition Elements 2 Chemistry of Ti V Cr Mn Fe and Co in various oxidation states excluding their metallurgy

Recent progress in separation of technetium-99 from spent nuclear fuel and radioactive waste. Challenges and prospects - ScienceDirect

The permanganate ion MnO-⁴ is deep purple but TcO4- and ReO4- are colourless, explain. – chemistrytutor.com
What is the reason for color of KMnO₄? - Quora
Answered: - MnO4 (aq) The purple color of MnO4…












![SOLVED: [MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2.](https://cdn.numerade.com/ask_images/3e1a1108c21446f7b6e87249991125fc.jpg)