Solved What is the equilibrium constant (Kp) at 45 °C for
4.9 (403) · $ 13.99 · In stock
Answer to Solved What is the equilibrium constant (Kp) at 45 °C for
A complex equilibrium question
The equilibrium constant (K) for the reaction,2SO2(g)+O2(g)2S03(g) at 1000 K is 3.5 atmWhat would be the partial pressure of oxygen gas,if the equilibrium is found to have equal moles ofSO2 and SO3?

chem 112 exam 2 Flashcards
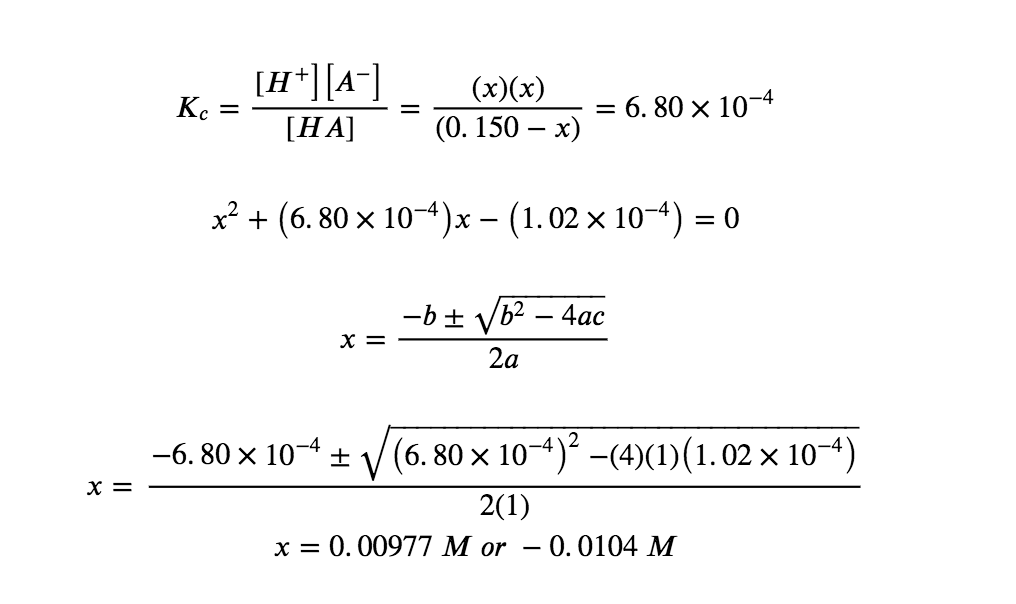
4.3 – Solving Equilibrium Problems – General Chemistry for Gee-Gees

The equilibrium constant `K_(c)` for the following reaction at `842^(@)`C is `7.90xx10^(-3)`. What i
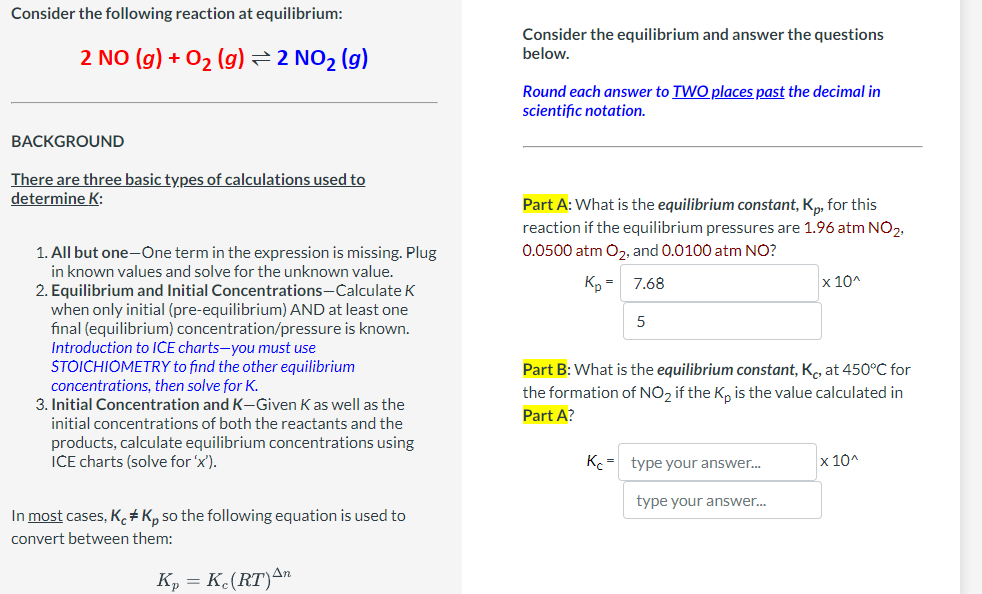
Solved What is the equilibrium constant, Kc, at 450°C for
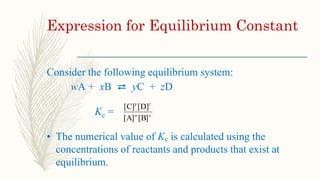
image.slidesharecdn.com/equilibrium-constant-prese
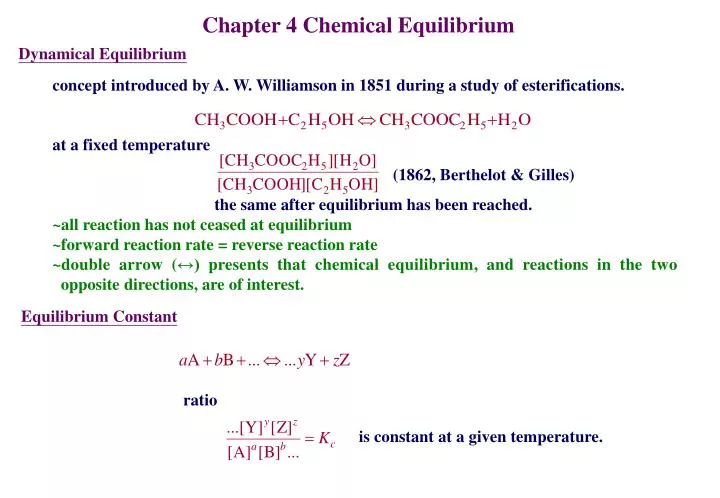
PPT - Chapter 4 Chemical Equilibrium PowerPoint Presentation, free download - ID:6955543

For the reaction, A(g) + B(g)rightarrow C(g) + D(g), Delta H^o and Delta S^o are, respectively, -29.8 kJ mol^{-1} and -0.100 kJ K^{-1} mo1^{-1} 298 K. The equilibrium constant the reaction 298
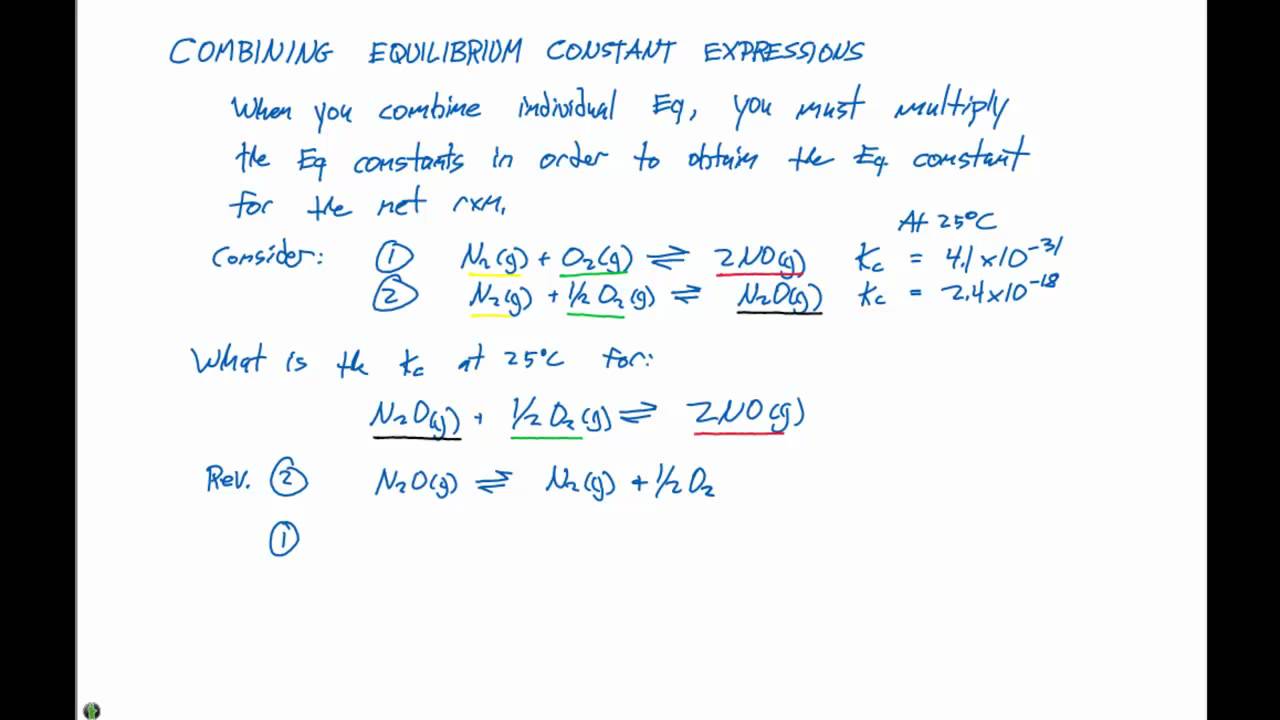
15.3 Combining Equilibrium Constants
✓ Solved: At 2200^∘ C, Kp=0.050for the reaction N2(g)+O2(g) ⇌ 2 NO(g) What is the partial pressure of












