- Home
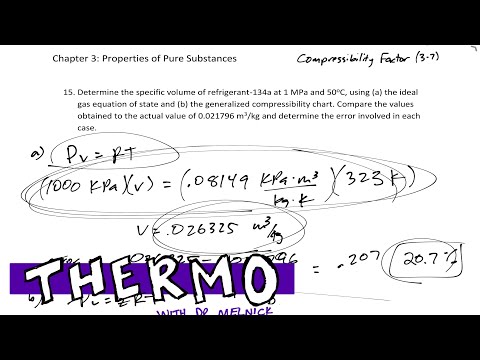
- compressibility factor equation
- 1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
4.8 (605) · $ 12.00 · In stock

Boyle Temperature for Van der Waals, Berthelot and Dieterici, Unit 2, BPC Class

Solved Worksheet: Gas Laws BACKGROUND In this class, we

Van Der Waals Equation of State - an overview
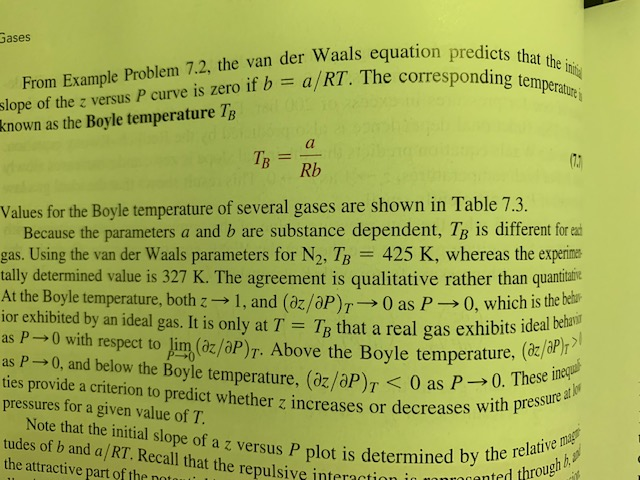
Solved the Boyle temperature TB is an important parameter
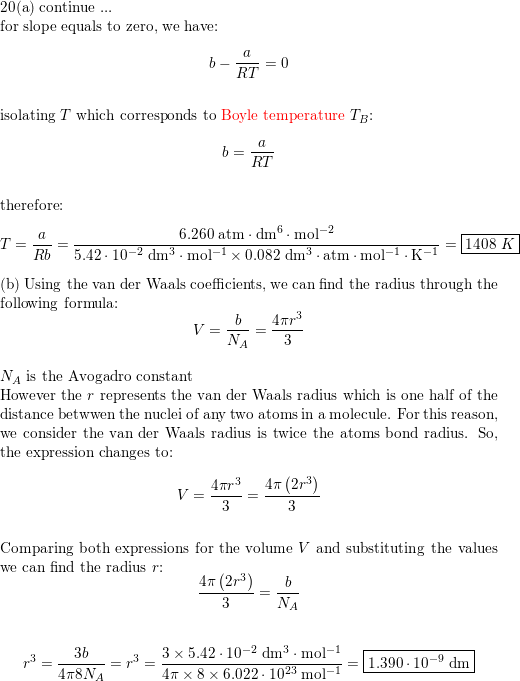
molecule regarded as a sphere. (b) Use the van der Waals parameters for hydrogen sulfide of the Resource section to calculate approximate values of (i) the Boyle temperature of the gas and (ii) the radius of a

The Boyle Temperature in a two-term virial equation of state
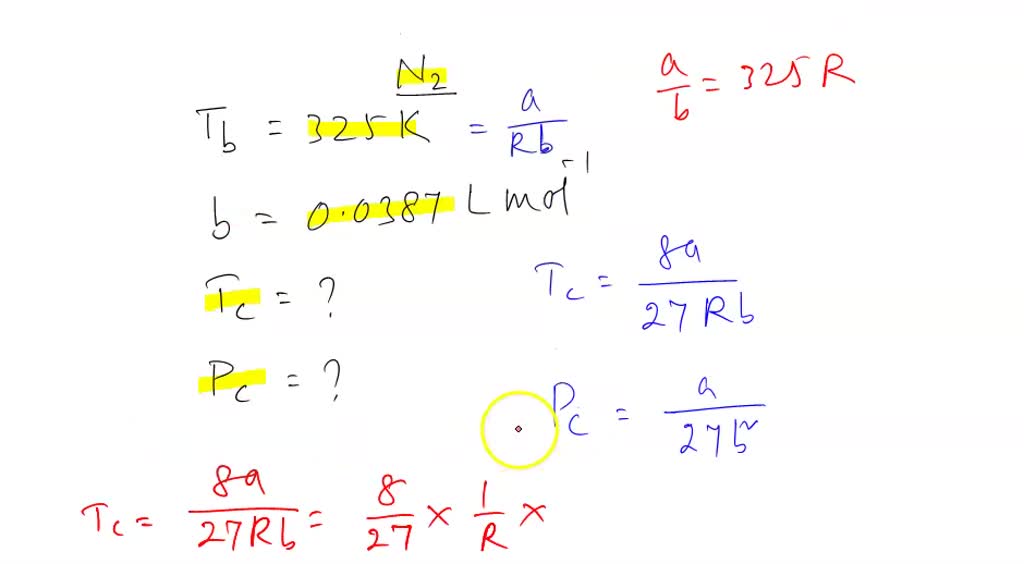
SOLVED: The Boyle temperature of N2(g) is 325 K and its van der Waals b parameter is 0.0387 L mol-1 . Calculate its critical temperature (K) and critical pressure (bar).

Solved The van der Waals equation of state can be used to

Derivation of Boyle Temp from real Gas Equation Lecture Note-31 Class XI Chemistry
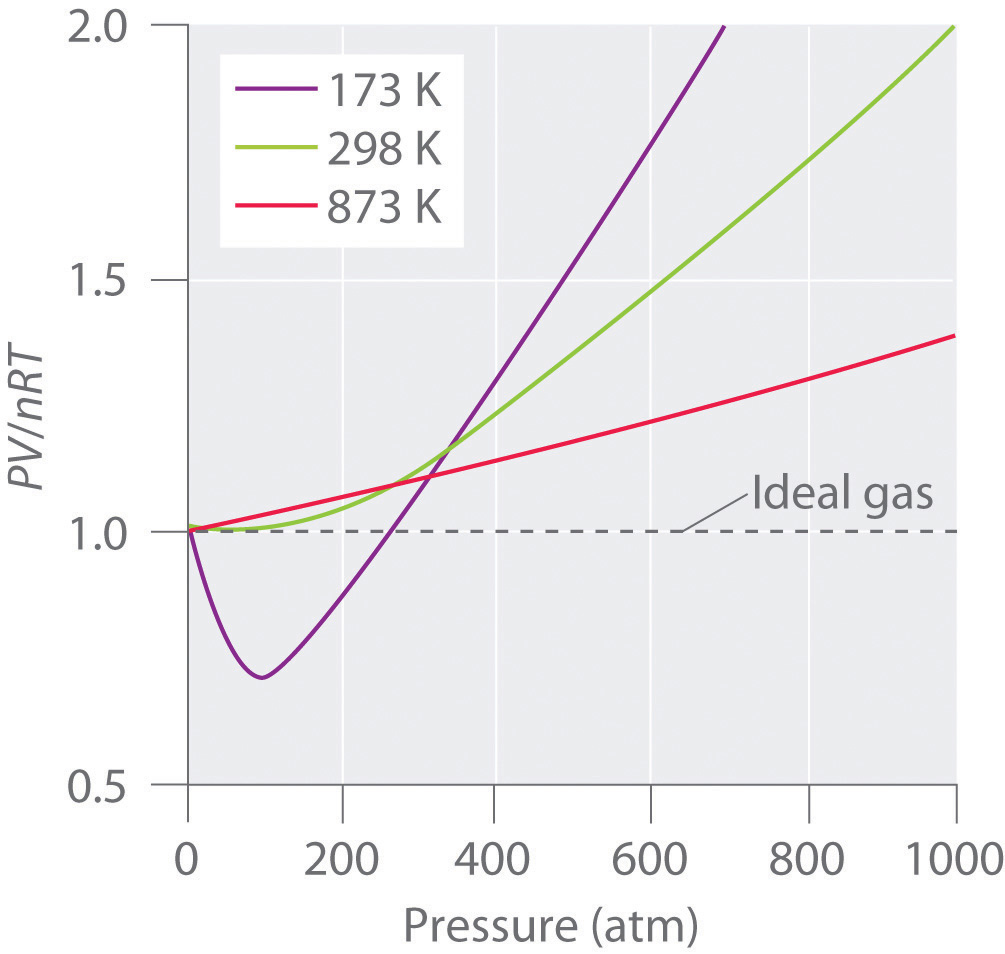
Chapter 11.1: Real Gases - Chemistry LibreTexts
How is Boyle's temperature related to Van Der Waal's constant? - Quora

Full PDF, PDF, Cell Nucleus