- Home
- compressibility factor equation
- If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect
If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect
4.6 (750) · $ 4.50 · In stock
If Z is a compressibility factor, van der Waals
If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

If Z is a compressibility factor, van der Waals equation at low pressure ..

2. 2. 1.000 a) 1.060.2 At low pressure the van der Waal's equation is reduced to [2017] (a) Z-PET LOVE Z VRT (c) pVm= RT (d) z = DI TIPA RT RT
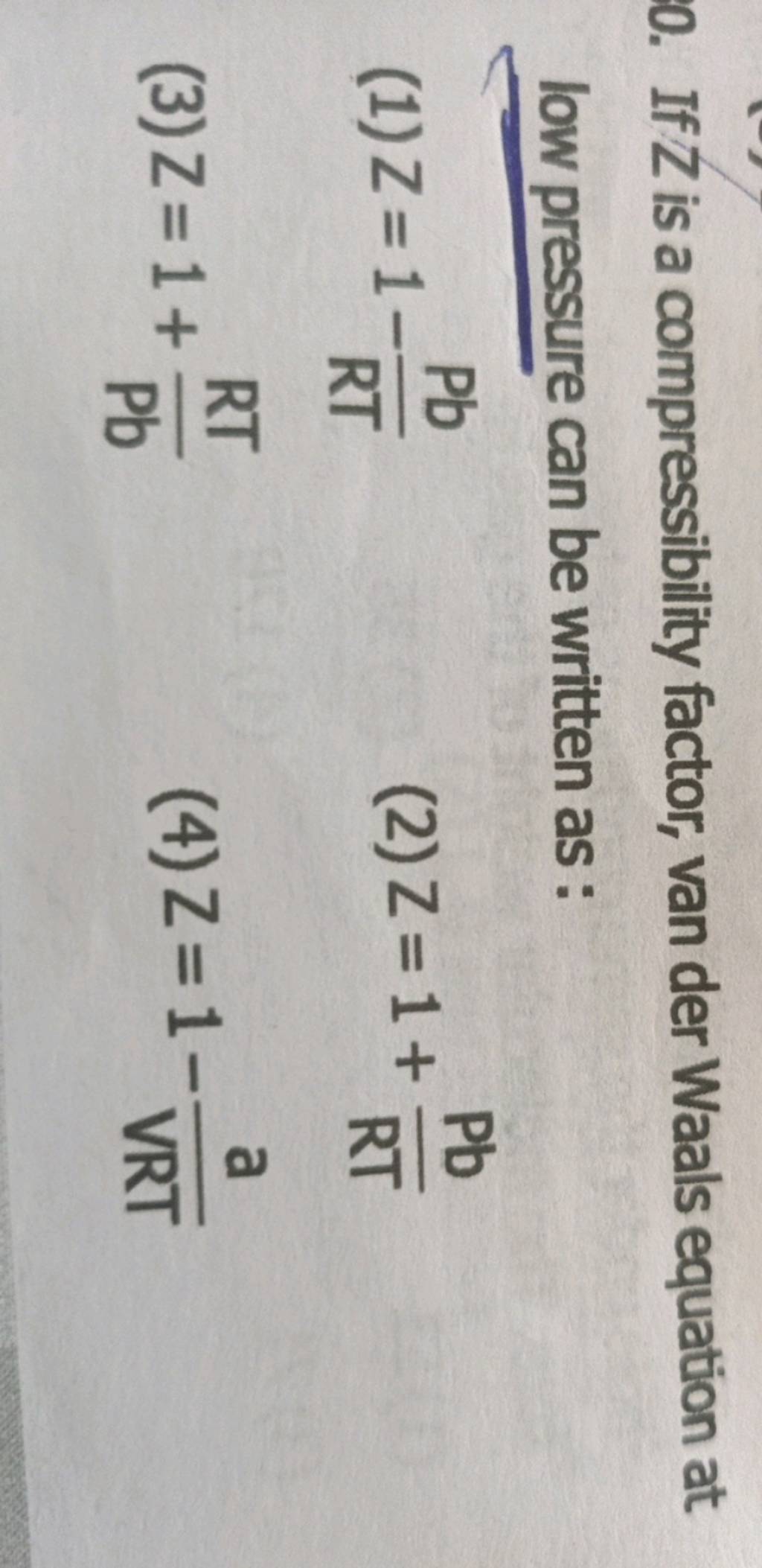
If Z is a compressibility factor, van der Waals equation at low pressure ..
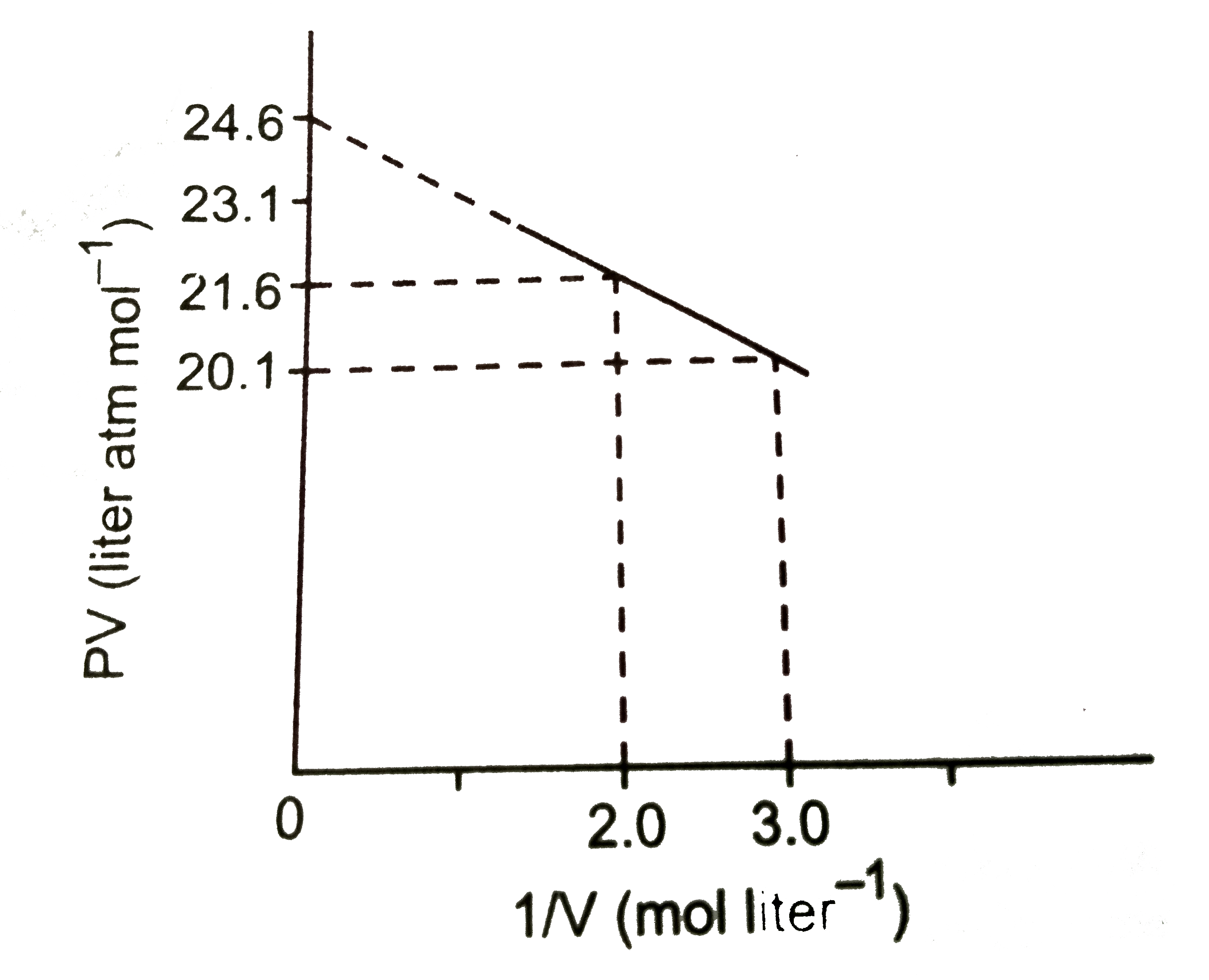
If Z is a compressibility factor, van der Waals' equation at low press
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

Compressibility factor (Z) for a van der Waals real gas at critical point is

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
Derive the expression for the pressure exerted by gas (Derive the Kinetic gas equation PV = 1/3 mn^2) - Sarthaks eConnect
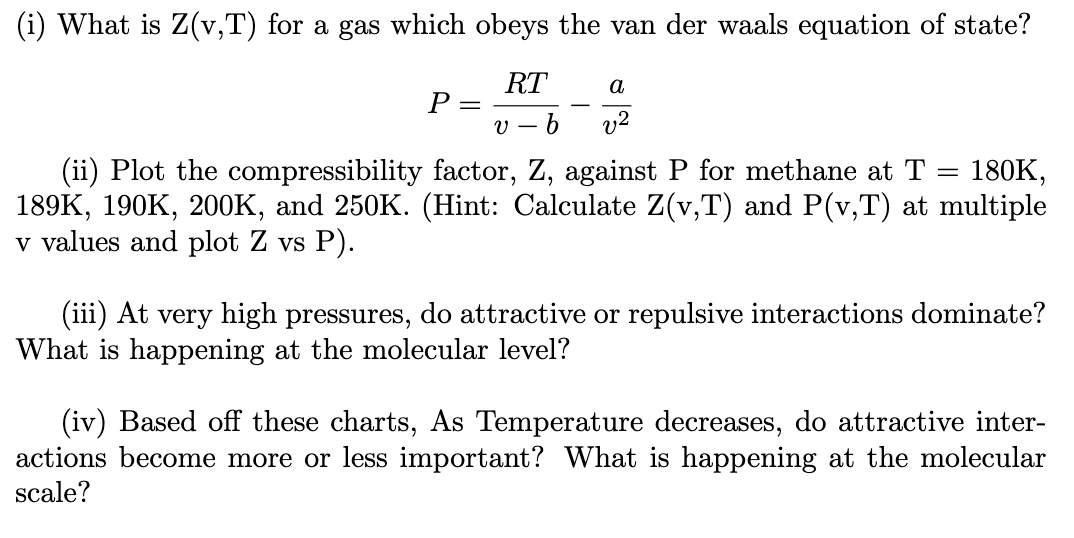
Solved (i) What is Z(v,T) for a gas which obeys the van der
The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, - Sarthaks eConnect

Solved 2. (20 points) At low pressures, the compressibility

The compressibility factor is Z = PV/R_g T. Evaluate
If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect









